Comprehensive Guide To Understanding The Structure And Properties Of Atoms
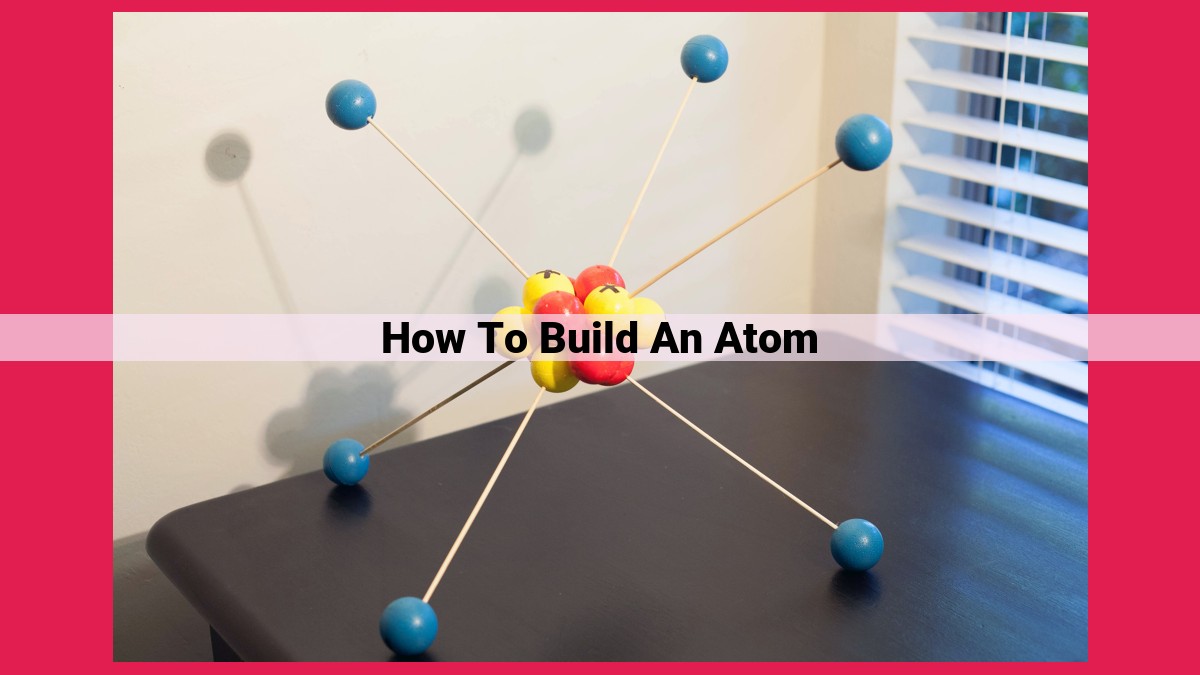
To build an atom, start with the nucleus, the core containing protons and neutrons. Protons determine the element and atomic number. Neutrons contribute to mass. Electrons orbit the nucleus in configured energy levels, with valence electrons influencing reactivity. The atomic number equals the number of protons, distinguishing elements, while the mass number indicates the total nucleons. Isotopes are atoms with different neutron counts. Electron configuration describes the electron arrangement, while valence electrons dictate chemical behavior. Atomic mass is the weighted average of isotope masses. Ions are atoms with altered electron counts, acquiring positive or negative charges.
The Nucleus: The Atom’s Inner Sanctum
Imagine the atom as a miniature solar system, with a dense, central core called the nucleus. The nucleus is where the atom’s vast majority of mass is concentrated, and it’s home to protons and neutrons.
Protons are positively charged particles that determine an atom’s element and its position on the periodic table. The number of protons is called the atomic number, and it’s the identity card of an element. For example, all atoms with one proton are hydrogen, while atoms with two protons are helium, and so on.
Neutrons are neutral particles that add to the mass of an atom but do not affect its element. The number of protons plus the number of neutrons equals the mass number. Atoms of the same element can have different numbers of neutrons, forming isotopes. Isotopes have the same number of protons but differ in mass.
Protons: The Identity Markers of Atoms
In the bustling atomic metropolis, protons take center stage as the key players determining an atom’s identity and its unique place in the periodic table. Imagine a bustling city, with each building representing an element and its inhabitants as the atoms residing within. The number of protons in an atom’s nucleus, like the address on a building, dictates which element it belongs to and assigns it a specific location on this tabular map of the atomic world.
Protons and Atomic Number:
Protons carry a positive electrical charge and reside in the heart of an atom, the nucleus. The number of protons in an atom’s nucleus defines its atomic number, which is like the identity card for that particular atom. Each element in the periodic table has a unique atomic number. Hydrogen, the simplest element, has a single proton, while Uranium, the heaviest natural element, boasts an atomic number of 92.
The Periodic Table’s Guiding Force:
The periodic table organizes elements based on their atomic numbers. Elements with similar chemical properties are grouped together in vertical columns, known as groups or families. The atomic number determines an element’s position in the periodic table, revealing patterns in the properties of different elements. Elements in the same group share similar electron configurations and thus exhibit comparable chemical behaviors.
Forging the Identity of an Atom:
The atomic number of an atom is its defining characteristic. It determines the element’s name, chemical symbol, and position on the periodic table. Without protons, atoms would be indistinguishable; they would all be a uniform sea of particles with no unique identities. Protons, therefore, serve as the architects of atomic diversity, shaping the chemical tapestry of the universe.
Protons, the positive charges dwelling in the nucleus, are the identity markers of atoms. They determine an atom’s element and assign it a specific address on the periodic table. Protons guide the properties of elements, shaping the chemical interactions that drive the universe. Without protons, the atomic landscape would be a chaotic realm, devoid of the organized structure and beauty that make chemistry possible.
Neutrons: Adding Mass and Shaping Isotopes
In the heart of every atom lies the nucleus, a bustling hub where protons and neutrons dance together. While protons proudly determine an atom’s identity, neutrons play a quiet yet crucial role in shaping its personality. These enigmatic particles contribute to the mass of an atom, influencing its atomic weight and giving rise to the fascinating world of isotopes.
Isotopes are atoms of the same element that share the same number of protons, but differ in the number of neutrons. This subtle difference in neutron count manifests itself in the mass number of the atom – the sum of protons and neutrons.
Imagine two neighboring atoms of the element carbon. Both possess six protons, the defining characteristic of carbon. However, one atom has six neutrons, while the other has seven. These atoms are isotopes of carbon: carbon-12 and carbon-13.
Carbon-12, the more abundant isotope, has a mass number of 12. This means it has six protons and six neutrons. In contrast, carbon-13 has a mass number of 13 due to its higher neutron count.
Isotopes can play significant roles in nature and technology. For instance, carbon-14, an isotope with eight neutrons, is used in radiocarbon dating to determine the age of organic materials. Isotopes also find applications in medicine, industry, and nuclear energy.
In conclusion, neutrons, though often overshadowed by protons, are indispensable contributors to the mass of atoms. Their varying presence gives rise to isotopes, captivating variations of the same element that shape the diverse tapestry of matter.
Electrons: The Orbiting Influencers
In the captivating realm of atoms, electrons reign supreme as the architects of chemical properties. They dance around the nucleus, like planets orbiting a star, in distinct energy levels. This delicate dance governs an atom’s ability to interact with others, shaping its chemical destiny.
Electron Configuration: A Blueprint of Reactivity
An atom’s electron configuration describes the arrangement of its electrons within these energy levels. It serves as a blueprint, determining the atom’s chemical fingerprint. The number of electrons in the outermost energy level, known as valence electrons, is particularly crucial. These electrons are the gatekeepers of chemical reactions, eagerly forming bonds with other atoms.
Electrons and Chemical Bonding: The Dance of Attraction
Valence electrons possess a remarkable ability to either gain or lose electrons. This exchange or sharing of electrons leads to the formation of chemical bonds, the fundamental force that holds atoms together. Atoms with a high number of valence electrons are more inclined to form bonds, while those with few valence electrons are less reactive.
Predicting Reactivity: A Chemical Puzzle
By understanding an atom’s electron configuration, we can predict its chemical behavior with surprising accuracy. For instance, metals typically have low ionization energies, indicating that they readily lose valence electrons to achieve stability. Nonmetals, on the other hand, have high ionization energies and prefer to gain electrons to establish a stable configuration.
Impact on Properties: Shaping the Chemical Landscape
The electron configuration of an atom not only influences its reactivity but also shapes its physical and chemical properties. For example, metals with loosely held valence electrons exhibit high electrical conductivity, while nonmetals with tightly held valence electrons are poor conductors of electricity.
Electrons, the tiny inhabitants of atoms, play a pivotal role in determining the chemical properties and behavior of matter. Their dance around the nucleus orchestrates the formation of bonds, shaping the molecular landscape that we inhabit. By delving into the electron configuration, we unlock the secrets of atomic reactivity, paving the way for a deeper understanding of the chemical world.
Atomic Number: The Unique Fingerprint of Elements
In the vast realm of matter, each atom possesses a distinct identity, an unyielding characteristic that sets it apart from its counterparts. This defining trait is captured by the concept of atomic number, a fundamental property that serves as the fingerprint of every element in the periodic table.
The atomic number represents the number of positively charged protons residing within an atom’s nucleus. These protons contribute to the core identity of an element, dictating its position on the periodic table and its unique chemical properties. Each element boasts a unique atomic number, ranging from 1 for hydrogen to 118 for oganesson.
In the periodic table, elements are arranged in ascending order of atomic number, creating a visual representation of their increasing proton count. This arrangement highlights the periodic trends in element properties, revealing the gradual changes in their chemical behavior and reactivity.
Understanding atomic number is crucial for comprehending the fundamental nature of elements. It allows scientists and researchers to predict and explain the chemical interactions and behavior of different elements, paving the way for advancements in fields such as chemistry, materials science, and nuclear physics.
Mass Number: Tallying the Nucleons
Deep within the heart of every atom lies a tiny yet mighty nucleus, where protons and neutrons reside in perfect harmony. Together, these particles contribute to the atom’s mass, a crucial property that distinguishes one element from another. The mass number, a numerical value, represents the total number of protons and neutrons packed within the nucleus.
Each proton, with its positive charge, contributes one unit of mass. Neutrons, on the other hand, carry no charge and contribute an additional unit of mass. By summing the number of protons and neutrons, we obtain the mass number.
For example, the most common isotope of hydrogen, hydrogen-1, has a mass number of one. This is because it contains just a single proton and no neutrons. In contrast, carbon-12, the most abundant isotope of carbon, has a mass number of 12. It boasts six protons (accounting for its atomic number) and six neutrons.
The mass number provides valuable insights into the atom’s structure and identity. A larger mass number indicates that the atom contains more nucleons (protons and neutrons), resulting in a heavier atom. Conversely, a smaller mass number corresponds to a lighter atom with fewer nucleons.
Understanding the mass number is essential for comprehending the diversity of elements and their properties. It forms the foundation for determining an atom’s mass and its position on the periodic table. Moreover, it helps us unravel the complexities of nuclear reactions and the behavior of atoms in chemical interactions.
**Isotopes: Variations on a Theme**
In the atomic realm, where the fundamental building blocks of matter reside, atoms play a pivotal role. Atoms, the smallest units of an element that retain its chemical identity, are like tiny universes with their own intricate structure. At the heart of every atom lies a microscopic nucleus, the central core that houses the protons and neutrons—the particles that define an element’s atomic number and mass.
The story of isotopes, however, unfolds in the realm of the neutron—the uncharged, subatomic particle that resides alongside protons within the nucleus. While protons determine an atom’s element and its unique position on the periodic table, neutrons influence its mass.
Atoms of the same element can possess varying numbers of neutrons, giving rise to different isotopic forms of that element. These isotopes share the same atomic number, meaning they contain the same number of protons, but they differ in neutron count and, consequently, mass number. The mass number is the sum of protons and neutrons in an atom’s nucleus.
Isotopes, with their distinctive neutron counts, provide valuable insights into the diversity of atomic structures. They play a crucial role in fields such as medicine, where radioactive isotopes are harnessed for diagnostic and therapeutic purposes. In archaeology, isotopic analysis helps determine the age and origin of artifacts. Isotopes also find applications in environmental studies, allowing scientists to trace the movement of pollutants and contaminants in the ecosystem.
The concept of isotopes underscores the intricate nature of the atomic world, where subtle variations within the nucleus give rise to distinct isotopic forms of the same element. These variations, though seemingly minor, have profound implications, influencing an atom’s mass and opening up a wealth of scientific applications.
Electron Configuration: A Dance of Energy Levels
At the heart of every atom lies a fascinating realm of energy levels, where electrons dance around the nucleus. The arrangement of these electrons, known as the electron configuration, plays a pivotal role in shaping the atom’s behavior.
Energy Levels
Picture an atom’s nucleus as a stronghold, surrounded by concentric energy levels, akin to the orbits of planets around the sun. Each energy level can accommodate a specific number of electrons, much like a celestial sphere holds a set number of celestial bodies.
Electron Arrangement
Electrons fill these energy levels from the lowest to the highest, as if following an invisible cosmic order. The first energy level, closest to the nucleus, can hold only two electrons, while the second can accommodate eight. Subsequent energy levels have increasingly higher capacities.
Valence Electrons
The electrons that occupy the outermost energy level are called valence electrons. These mobile electrons are like the social butterflies of the atomic world, eager to interact with neighboring atoms. The number of valence electrons dictates the atom’s chemical activity and its ability to form bonds with other atoms.
Significance
Electron configuration is a fundamental property that determines an atom’s chemical and physical characteristics. It influences an atom’s reactivity, bonding behavior, magnetism, and even its color. By understanding how electrons are arranged in energy levels, we can predict and explain the properties of various elements and compounds.
The electron configuration of an atom is a subtle yet profound choreography of energy. It’s a dance of electrons that orchestrates the atom’s behavior and plays a crucial role in the intricate tapestry of chemical interactions that shape our world.
Valence Electrons: The Chemistry Catalysts
In the world of atoms, the dance of electrons holds the key to chemical reactions. Like tiny magnets, these electrons orbit the atom’s nucleus, but it’s the ones on the outermost level, known as valence electrons, that play a pivotal role in determining an atom’s destiny.
These valence electrons are like the social butterflies of the atom, eager to interact with others. It’s their availability that drives an atom’s ability to form bonds, the chemical connections that hold molecules together.
Imagine an atom as a group of teenagers at a party. Valence electrons are the outgoing ones, ready to mingle and forge connections with other atoms. The more valence electrons an atom has, the more bonds it can form.
This dance of valence electrons is what gives rise to the periodic table. Elements in the same group share the same number of valence electrons, which explains why they have similar chemical properties. For instance, fluorine and chlorine both have seven valence electrons, making them highly reactive and often found in compounds.
The interplay of valence electrons is like a symphony, with each atom playing its own unique tune. It’s these interactions that orchestrate the vast array of molecules and materials that make up our world. From the proteins in our bodies to the plastics in our gadgets, the dance of valence electrons weaves the fabric of our existence.
Atomic Mass: A Weighted Average
Every atom, the fundamental building block of matter, possesses a distinct atomic mass, a numerical value that reflects its overall heaviness. However, the atomic mass of an element is not a fixed number but rather a weighted average, taking into account the contributions of its various isotopes.
Isotopes are different forms of the same element, sharing the same number of protons but differing in the number of neutrons. These variations in neutron count lead to different mass numbers, essentially the total number of protons and neutrons within the nucleus.
The atomic mass of an element represents the average mass of all its isotopes, weighted by their relative abundance. Each isotope contributes its mass, multiplied by its percentage occurrence, to this weighted calculation. This weighted average mass provides a more accurate representation of the element’s overall mass than the mass number of any single isotope.
For instance, the element chlorine has two naturally occurring isotopes: chlorine-35 and chlorine-37. Chlorine-35 accounts for approximately 76% of all chlorine atoms, while chlorine-37 makes up the remaining 24%. Chlorine-35 has a mass number of 35, while chlorine-37 has a mass number of 37.
Using a weighted average formula, the atomic mass of chlorine can be calculated as:
Atomic mass = (Mass number of isotope 1 × Abundance of isotope 1) + (Mass number of isotope 2 × Abundance of isotope 2) + ...
Plugging in the values for chlorine:
Atomic mass = (35 × 0.76) + (37 × 0.24) = 35.45
Therefore, the atomic mass of chlorine is 35.45, which represents the average mass of all chlorine atoms, considering the contributions of both chlorine-35 and chlorine-37. This weighted average provides a more precise estimation of the element’s overall heaviness.
Ions: Charged Atoms in Motion
In the bustling world of atoms, there exists a fascinating category known as ions. These are atoms that have undergone a dramatic makeover, with their electron counts altered, leaving them with a newfound charge. This transformation from neutral atoms to charged particles unlocks a realm of exciting chemical reactions and plays a pivotal role in biological processes.
Just like protons and neutrons reside in the heart of the atom, the nucleus, electrons dance around it in designated energy levels. When an atom loses or gains electrons, its once-balanced electrical charge is thrown into disarray. Positively charged ions are born when an atom loses one or more electrons, while negatively charged ions emerge when it gains additional electrons.
The presence of ions in our world is far from trivial. They are not mere spectators but active participants in countless chemical reactions. Ions can eagerly react with one another, forming ionic bonds that hold molecules together. They also play a crucial role in electrolysis, a process that uses electricity to drive chemical reactions, and electroplating, the art of coating one metal with another.
But the influence of ions extends beyond chemistry. In the realm of biology, ions are indispensable for maintaining delicate balances within living organisms. In our bodies, ions regulate nerve impulses, control muscle contractions, and even influence our heartbeat. A prime example is the sodium-potassium pump, a molecular machine that diligently maintains the proper balance of sodium and potassium ions across cell membranes.
Understanding ions is not merely an academic pursuit; it holds practical relevance in our daily lives. The batteries that power our devices rely on the movement of ions to generate electricity. Ion exchange resins are employed in water purification systems to remove impurities. And ion propulsion systems propel spacecraft through the vastness of space.
In conclusion, ions are not just charged atoms; they are dynamic entities that orchestrate a myriad of chemical reactions and biological processes. From the batteries that light up our homes to the intricate workings of our bodies, ions play an indispensable role in shaping our world.





