Percentage Composition By Mass: Uncover The Elemental Makeup Of Compounds
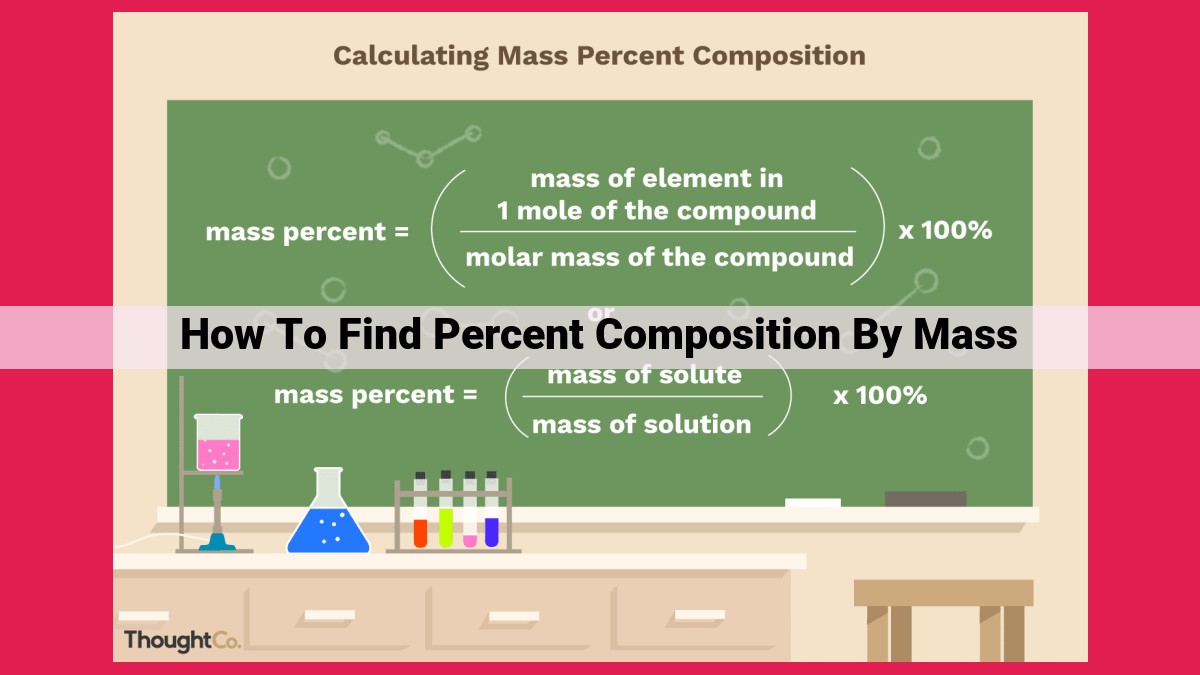
Percent composition by mass is a crucial concept in chemistry that reveals the elemental makeup of compounds. It involves calculating the mass percentage of each element in a compound based on its total mass. The process entails determining the mass of each element, calculating the total compound mass, and then dividing the individual element’s mass by the total mass and multiplying by 100. Percent composition enables the identification and characterization of compounds, aids in determining empirical formulas, and provides insights into their molecular composition.
Percent Composition by Mass: Understanding the Makeup of Compounds
In the realm of chemistry, understanding the composition of compounds is paramount. Percent composition by mass emerges as a crucial tool, providing a quantitative measure of the elements present within a compound. This concept illuminates the elemental proportions, revealing the essence of the compound and its unique properties.
Percent composition by mass plays a pivotal role in chemical analysis, allowing scientists to:
- Accurately identify and characterize compounds by comparing their percent compositions.
- Determine the empirical formula of a compound, providing insights into its elemental ratios.
Furthermore, percent composition by mass finds applications in diverse fields, ranging from pharmaceutical analysis to environmental monitoring. By unraveling the elemental makeup of various substances, it empowers scientists to develop new materials, optimize industrial processes, and safeguard the environment.
Fundamental Concepts
- Explain the terms “mass of each element” and “total mass of the compound.”
Fundamental Concepts: Understanding the Building Blocks of Percent Composition
In chemistry, understanding the composition of substances is crucial. Percent composition by mass provides a precise way to quantify the relative amounts of elements within a compound. Before diving into the calculations, let’s establish a solid foundation by clarifying two key terms:
-
Mass of each element: This refers to the total mass of a specific element present in the compound. It’s measured in grams (g).
-
Total mass of the compound: This is the combined mass of all the elements present in the compound. It’s also expressed in grams.
These two values form the basis of percent composition by mass. By comparing the mass of each element to the total mass, we can determine the relative proportions of elements within the compound, helping us understand its chemical composition.
Calculating Percent Composition by Mass
In the realm of chemistry, understanding the composition of compounds is crucial. Percent composition by mass provides a quantitative method to determine the relative amounts of elements present in a compound, making it an indispensable tool for chemical analysis.
To calculate percent composition by mass, we embark on a three-step journey:
-
Determine the Mass of the Element: First, we need to measure the mass of each individual element within the compound. This involves examining the chemical formula and multiplying the atomic mass of each element by its respective number of atoms in the formula.
-
Calculate the Total Mass of the Compound: Next, we calculate the total mass of the compound by adding up the masses of all the elements present. This value serves as the denominator in our percent composition equation.
-
Calculate the Percent Composition: Finally, we calculate the percent composition of each element by dividing its mass by the total mass of the compound and multiplying by 100. The result represents the percentage contribution of that element to the compound’s overall mass.
For example, consider sodium chloride (NaCl). Sodium has an atomic mass of 22.99 g/mol, while chlorine has an atomic mass of 35.45 g/mol. The chemical formula of NaCl indicates one sodium atom and one chlorine atom.
- Mass of sodium (Na): 22.99 g/mol
- Mass of chlorine (Cl): 35.45 g/mol
- Total mass of NaCl: 22.99 g/mol + 35.45 g/mol = 58.44 g/mol
Percentage composition of sodium: (Mass of Na / Total mass of NaCl) x 100 = (22.99 g/mol / 58.44 g/mol) x 100 = 39.34%
Percentage composition of chlorine: (Mass of Cl / Total mass of NaCl) x 100 = (35.45 g/mol / 58.44 g/mol) x 100 = 60.66%
Percent composition by mass not only provides valuable insights into the composition of compounds but also has practical applications in fields such as:
- Identifying and characterizing compounds: By comparing the percent composition of unknown compounds to known values, scientists can determine the identity of the compound.
- Determining the empirical formula of a compound: Percent composition data can be used to establish the simplest whole-number ratio of elements in a compound, providing an empirical formula.
Calculating Percent Composition by Mass: A Step-by-Step Example
In the realm of chemistry, understanding the composition of compounds is crucial, and that’s where percent composition by mass comes into play. Imagine you’re holding a bag of sugar, eager to unravel its chemical secrets. Well, calculating the percent composition by mass is like baking a cake – it’s all about measuring ingredients and figuring out their proportions.
Step 1: Determine the Mass of Each Element
Just as you would weigh flour in a cake recipe, we need to determine the mass of each element present in our compound. Let’s say our sugar (C12H22O11) contains 12 grams of carbon, 22 grams of hydrogen, and 32 grams of oxygen.
Step 2: Calculate the Total Mass of the Compound
Just as the total weight of your cake batter matters, we need to calculate the total mass of our sugar. In this case, we simply add the masses of each element: 12 g + 22 g + 32 g = 66 g.
Step 3: Calculate the Percent Composition
Now comes the fun part – finding the percent composition of each element. It’s like determining the percentage of chocolate chips in your cake batter. We divide the mass of each element by the total mass of the compound and multiply by 100.
For carbon, it’s: (12 g / 66 g) x 100 = 18.18%
For hydrogen, it’s: (22 g / 66 g) x 100 = 33.33%
For oxygen, it’s: (32 g / 66 g) x 100 = 48.48%
And there you have it! Our sugar is composed of 18.18% carbon, 33.33% hydrogen, and 48.48% oxygen. Think of it as the recipe for your favorite cake, with each element playing a unique role in its overall flavor and properties.
Applications of Percent Composition by Mass: Unveiling the Identities of Compounds
Percent composition by mass is an indispensable tool in chemistry that enables us to unravel the mysteries of compounds. It serves as a window into their composition, allowing us to identify and characterize them with remarkable precision.
Unveiling the Identities of Compounds
Percent composition by mass provides a unique chemical fingerprint for each compound. By meticulously measuring the mass of each element within a compound and expressing it as a percentage of the total mass, we can create a distinctive compositional profile. This profile can be compared to known compounds to identify or confirm the identity of the unknown substance.
Determining the Empirical Formula: A Path to Compound Description
Beyond identifying compounds, percent composition by mass also empowers us to determine their empirical formula. The empirical formula represents the simplest whole-number ratio of elements within a compound. By establishing the percent composition by mass, we can convert these percentages into mole ratios, which in turn guide us towards the empirical formula.
Armed with the knowledge of percent composition by mass, we can unlock the secrets of compounds. It becomes possible to identify their unique identities and determine their empirical formulas, opening doors to further understanding and countless applications in scientific research, industry, and everyday life.
**Further Considerations**
While percent composition by mass is a valuable tool, it’s essential to understand its limitations. Accuracy is crucial, as any errors in measuring the mass of elements or the total mass of the compound will impact the accuracy of the result. Moreover, this method assumes that the compound is pure and does not contain any impurities. In reality, many compounds may contain trace amounts of other elements, which can influence the percent composition.
Furthermore, percent composition by mass alone does not provide information about the molecular structure of the compound. For instance, two different compounds might have the same percent composition but different molecular structures, which can affect their chemical properties. To fully understand the nature of a compound, it’s necessary to determine its empirical formula, which represents the simplest whole-number ratio of elements present. While percent composition by mass can provide a starting point, further analysis is often required to establish the exact molecular structure.
Additionally, it’s important to note that percent composition by mass is a relative measurement. It only indicates the relative amounts of different elements within a compound, not the absolute amount present. To determine the absolute mass of each element, the total mass of the compound needs to be known.