The Ultimate Guide To Solutions: Composition, Properties, And Applications
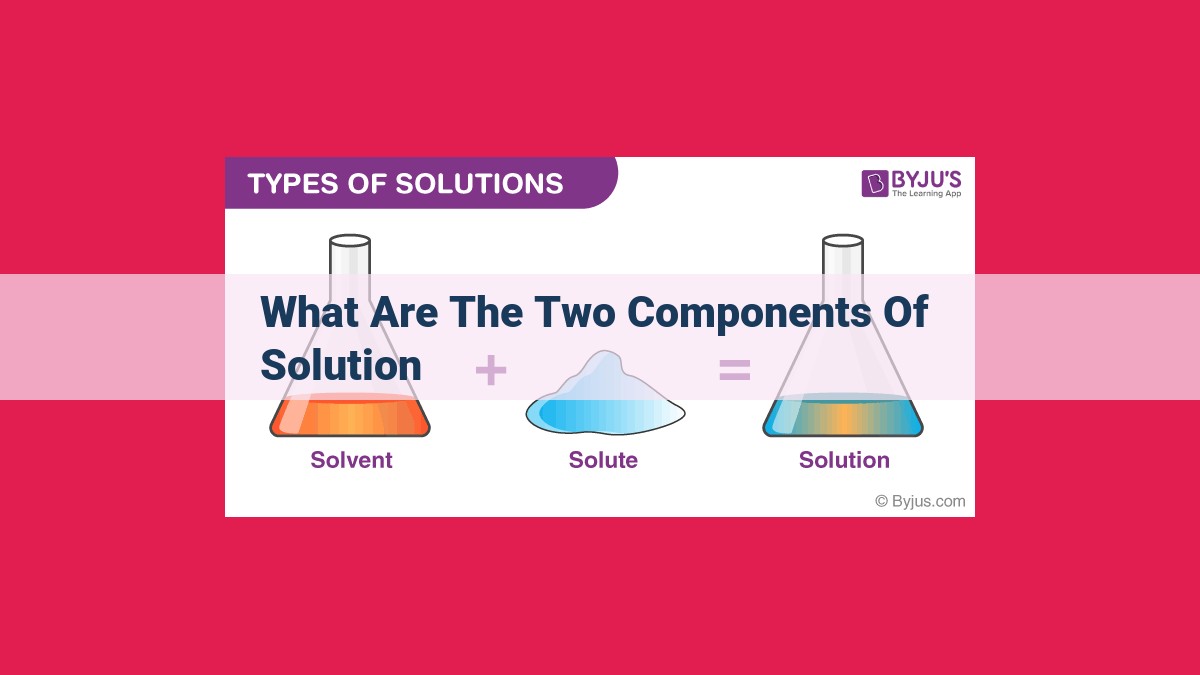
A solution is a homogeneous mixture consisting of two components: the solute and the solvent. The solute is the substance that is dissolved into the solvent, while the solvent is the substance that does the dissolving. The interaction between the solute and the solvent creates a uniform mixture with properties distinct from those of its individual components. The concentration of the solution, which refers to the relative amounts of solute and solvent, plays a crucial role in determining the solution’s properties.
Definition of a solution as a homogeneous mixture of multiple substances.
Components of a Solution: The Building Blocks of Mixtures
Welcome to the fascinating world of solutions, where multiple substances mingle harmoniously, forming a uniform entity. Understanding the components of a solution unlocks the secrets of these ubiquitous mixtures, empowering us to appreciate their diverse applications.
At the heart of a solution lies a homogeneous blend, where different substances coexist seamlessly. These substances can be solid, liquid, or gaseous, each playing a unique role in the solution’s makeup.
The two primary components of a solution are the solute and the solvent. Think of the solute as the guest, a substance that’s dissolved and dispersed evenly throughout the solution. The solvent, on the other hand, is the welcoming host, a substance that dissolves the solute and creates a uniform mixture.
The interaction between solute and solvent is a delicate dance, much like the harmony between yin and yang. The solvent’s ability to dissolve the solute depends on their chemical similarities, creating a soluble solution. When the solvent reaches its maximum capacity to accommodate the solute, the solution becomes saturated.
Significance of understanding the solution’s building blocks.
Solutions are ubiquitous in our world, from the air we breathe to the salt we sprinkle on our food. Understanding their components is crucial for comprehending their behavior and applications.
Significance of Understanding the Solution’s Building Blocks:
The components of a solution are not merely abstract concepts; they play a vital role in our understanding of these ubiquitous substances. By grasping the nature and interactions of these building blocks, we can unravel the secrets of solutions and harness their power for various purposes.
-
Predicting Solution Behavior: The components of a solution determine its physical and chemical properties. Understanding these components allows scientists and engineers to predict how a solution will behave under different conditions.
-
Optimizing Applications: The functionality of solutions depends heavily on their components. Whether it’s developing new cleaning agents, designing innovative drugs, or creating sustainable energy sources, understanding the building blocks of solutions empowers us to optimize their applications.
-
Understanding Biological Processes: Many biological phenomena involve solutions, such as the exchange of nutrients in cells and the regulation of bodily fluids. Grasping the components of solutions provides insights into these intricate biological processes.
Components of a Solution: Unveiling the Secrets of Mixture
In the realm of chemistry, a solution emerges as a captivating dance between substances, where one elegantly dissolves into another, forming a harmonious blend. Understanding the building blocks of a solution, the solute and the solvent, holds the key to unlocking the mysteries of this captivating phenomenon.
Let’s focus on the enigmatic solute, the substance that embarks on an extraordinary journey of dissolving into a solvent. Imagine a tiny particle of solute, like a grain of salt, bravely venturing into the embrace of a solvent, such as water. The solvent, like a benevolent host, welcomes the solute into its molecular embrace, enveloping it in a comforting embrace.
As the solute dissolves, it undergoes a remarkable transformation. It relinquishes its solid or liquid form, surrendering to the fluidity of the solvent. The result is a homogeneous mixture, where the solute uniformly permeates the solvent, leaving no trace of its former physical state. This intimate mingling gives rise to a concentration, a measure of the amount of solute dissolved in a given amount of solvent. Concentration plays a pivotal role in determining the solution’s properties and its behavior in various applications.
The tale of the solute and solvent is a testament to the power of interaction, a harmonious dance that gives birth to a new entity with unique characteristics. Together, they form the cornerstone of solutions, captivating mixtures that play a vital role in science, industry, and our everyday lives, from the salt that seasons our food to the medicines that heal our bodies.
Components of a Solution: Unraveling the Building Blocks of Unity
In the world of chemistry, a solution stands as a testament to the harmonious coexistence of multiple substances. This homogeneous mixture, like a symphony, requires a delicate balance between its components to achieve its unique properties. To fully appreciate the wonders of a solution, let’s embark on a journey to understand its building blocks.
Solute: The Guest of Honor
At the heart of a solution lies the solute, the substance that plays the guest role, dissolving within the embrace of another. Imagine a tea party where the solute is the sugar crystals, dissolving gracefully into a warm cup of tea. The mixture, known as a solution, forms when the solute particles disperse evenly throughout the solvent. Concentration, a measure of the solute’s presence, plays a pivotal role in shaping the solution’s characteristics.
Solvent: The Hospitable Host
Complementary to the solute’s presence is the solvent, the gracious host that welcomes the solute into its embrace. The solvent, a liquid in most cases, provides the medium for the solute to dissolve. Water, with its abundance and polarity, serves as a universal solvent, readily dissolving a wide range of solutes. As the solute dissolves, it interacts with the solvent molecules, forming a new entity with distinct properties.
Unveiling the Secrets of a Solution: Exploring the Components
In the realm of chemistry, a solution reigns supreme, being a harmonious concoction where multiple substances intermingle to form a uniform blend. To truly grasp the intricacies of this captivating mixture, we must delve into its fundamental building blocks.
One of the key players in this alchemical symphony is the solute, the substance that willingly surrenders its form and gracefully dissolves into the embrace of its companion, the solvent. Imagine a troupe of dancers, the solute, gracefully entwined within the rhythmic embrace of the solvent, the dance floor.
The proportion of solute to solvent in this harmonious union dictates the solution’s concentration. A concentrated solution boasts a vibrant presence of solute, while a dilute solution dances with a gentle touch of solute. These variations in concentration orchestrate a diverse symphony of solution properties, much like the varying tempos and melodies of a musical masterpiece.
The interplay between solute and solvent is a captivating dance of attraction and integration. The solvent’s molecules, like benevolent hosts, welcome the solute molecules into their midst, forming a cohesive ensemble. This intimate union grants the solution its distinctive characteristics, dictating its appearance, behavior, and potential applications.
In the vast canvas of our world, solutions abound. From the salty embrace of the ocean to the refreshing clarity of a mountain stream, solutions are ubiquitous. They play a vital role in our daily lives, serving as the foundation for everything from life-saving medicines to the beverages that quench our thirst.
By unraveling the intricate tapestry of a solution’s components, we gain invaluable insights into the fundamental principles of chemistry. These components – the solute, solvent, and their captivating dance – orchestrate a symphony of properties that shape our world.
Understanding the Building Blocks of a Solution: Solute and Solvent Interplay
When we dissolve a substance in another, we create a solution—a homogeneous mixture where the components are evenly distributed. Understanding the building blocks of a solution, solute and solvent, is crucial for grasping the nature of solutions.
The solute is the substance that’s dissolved, while the solvent is the substance that does the dissolving. The proportions of solute and solvent determine the solution’s mixture and concentration. A high concentration means there’s more solute relative to solvent, while a low concentration indicates a lesser amount of solute.
The interaction between solute and solvent is fascinating. The solute molecules disperse throughout the solvent, forming a uniform mixture. This process can alter the solvent’s properties, creating solutions with unique characteristics. For instance, dissolving salt in water increases its boiling point.
The nature of the solution also depends on the solvent-solute interaction. Some solvents readily dissolve certain solutes, forming miscible solutions. Others may exhibit immiscibility, where the solute and solvent form separate layers. Understanding these interactions is essential for predicting solution behavior and their applications.
The Enchanted Dance of Solute and Solvent
In the realm of chemistry, where substances intertwine to create wonders, we embark on a journey into the very essence of a solution: its components. The solute and solvent, like celestial bodies, dance in harmony to form a new entity, a solution.
The solute, the substance being dissolved, seeks refuge in the welcoming embrace of the solvent. As these chemical partners come into contact, the solvent’s molecules surround the solute particles, enveloping them in a gentle embrace. This embrace is not merely physical, but a profound chemical interplay.
The solute particles, eager to dissolve, release their inhibitions and shed their solid or gaseous form. The solvent, ever the gracious host, provides a protective shelter, cloaking the solute particles in a protective layer of its own molecules. Together, they create a homogeneous blend, a symphony of substances, where distinctions between solute and solvent fade into seamless unity.
The concentration of the solution, a measure of the solute’s presence within the solvent, plays a crucial role in shaping the solution’s properties. A concentrated solution hums with a higher population of solute particles, while a dilute solution offers them more space to roam freely.
This delicate balance between solute and solvent orchestrates the solution’s unique characteristics. The solubility of a solute, its ability to dissolve in a solvent of choice, is a testament to their harmonious connection. The strength of a solution, a reflection of its potency, speaks to the abundance of solute particles eagerly mingling with the solvent molecules.
As the solute and solvent dance their intricate pas de deux, they paint a vibrant canvas of possibilities. From the soothing relief of medicinal solutions to the sparkling cleanliness of household cleaners, the applications of solutions span the spectrum of science, industry, and everyday life.
In conclusion, the interplay of solute and solvent is a mesmerizing dance of chemistry, where the fusion of entities creates a new substance with remarkable properties. Understanding these components is not merely a scientific pursuit, but a testament to the harmonious collaborations that shape our world. May this exploration inspire you to unravel further mysteries of the chemical realm, as we continue to marvel at the enchantment of solutions.
Understanding the Components of a Solution: A Solute, Solvent, and Their Interplay
When we dissolve a substance in a liquid, we create a solution—a homogeneous mixture where the solutes and solvents become one. Understanding the components and their interaction is crucial for comprehending the diverse applications of solutions across various fields.
Solute: The Essence of a Solution
The solute is the substance that’s dispersed within the solvent. It’s the “substance of interest”, the one we want to understand or utilize within the solution. The amount of solute relative to the solvent determines the concentration of the solution, which has a significant impact on its properties and potential uses.
Solvent: The Medium of Dissolution
The solvent is the “carrier liquid” that dissolves the solute. It’s typically present in greater quantity and provides the environment for the solute to disperse and interact with. The nature of the solvent, such as its polarity and ability to form interactions with the solute, plays a crucial role in determining the solution’s characteristics.
The Dance Between Solute and Solvent
The interaction between solute and solvent is a fascinating dance. When the solute dissolves in the solvent, it forms a uniform mixture that’s indistinguishable from the pure solvent to the naked eye. This process involves the breaking of intermolecular bonds within the solute and the formation of new interactions between the solute and solvent molecules.
Concentration, a critical factor in this dance, affects solution properties such as freezing point, boiling point, vapor pressure, and osmosis. It directly influences the molecular interactions within the solution, ultimately shaping its behavior and applications.
Beyond the Theory: Practical Applications
Solutions are ubiquitous in our daily lives and numerous industries. From medical solutions that deliver drugs to our bodies to cleaning agents that remove stains, solutions play a diverse role. In science, they’re used as reaction media, titrating agents, and analytical tools.
Understanding the components and their interplay in solutions is not just an academic exercise but a practical tool that allows us to harness their potential and navigate the complex world of mixtures around us.
Components of a Solution: Unraveling the Building Blocks of Mixtures
In the realm of chemistry, understanding solutions is crucial for deciphering the behavior of matter. A solution, defined as a homogeneous mixture of multiple substances, forms the foundation of many natural and engineered systems. To grasp its complexities, we must delve into its fundamental building blocks.
Solute, the substance that undergoes dissolution, finds a home within the solvent, the substance that facilitates the dissolving process. The intricate interplay between solute and solvent gives rise to a mixture, the resulting solution. The concentration of the solute within the solvent dictates the extent of its presence.
When salt dissolves in water, it creates a saltwater solution, a common example of a solution. Similarly, sugar dissolves to form sugar solutions. Perhaps surprisingly, air itself is a solution of gases, with nitrogen comprising most of its volume.
The versatility of solutions extends far beyond these familiar examples. Medical solutions administer therapeutic substances into the body, while cleaning agents harness the power of solutions to remove dirt and grime. The food we eat is an edible symphony of solutions, from the flavorful broth of soups to the sweet delight of desserts.
Innumerable applications of solutions permeate our daily lives. They play a pivotal role in scientific research, industrial processes, and countless technological advancements. Understanding the intricacies of solutions empowers us to navigate the complexities of the world around us.
As we continue our exploration of solutions, we unravel the enigmatic dance between solute and solvent. From the depths of oceans to the heights of the atmosphere, solutions shape our planet and enrich our lives.
Solutions: The Versatile Building Blocks of Our World
Understanding the Components of a Solution
A solution is a homogeneous mixture of two or more substances, where one substance is dissolved in the other. The dissolved substance is called the solute, while the dissolving substance is called the solvent. Together, they form the foundation of various everyday and scientific applications.
Solute and Solvent: A Dynamic Duo
Solutes, typically solids, liquids, or gases, contribute unique properties to a solution. They determine the mixture’s concentration and can significantly alter its physical and chemical characteristics. On the other hand, the solvent plays a crucial role in dissolving the solute, forming a stable solution.
Interplay of Solute and Solvent
The interaction between solute and solvent is essential for solution formation. Solvents can weaken the intermolecular forces holding solutes together, allowing them to disperse evenly. The resulting concentration influences the solution’s properties, such as its boiling point, freezing point, and conductivity.
Real-World Examples of Solutions
Solutions are ubiquitous in nature and play a pivotal role in our lives. Salt water (aqueous sodium chloride solution) is a common example, while sugar solutions add sweetness to our beverages and desserts. Even the air we breathe is a solution of gases like nitrogen and oxygen.
Versatile Applications of Solutions
The applications of solutions extend far beyond everyday examples. In science, they are used for research, analysis, and experimentation. In industry, they serve as cleaning agents, solvents, and chemical reagents. In our daily lives, solutions are found in medications, cosmetics, and even food products, providing nourishment and flavor.
Understanding the components and their interplay in a solution is crucial for comprehending the behavior of many substances in our world. From the salt water we drink to the cleaning solutions we use, solutions shape our lives in countless ways. As we delve deeper into the fascinating world of solutions, we unlock the potential for innovation and a better understanding of the universe around us.
Components of a Solution: Understanding the Building Blocks of Homogeneous Mixtures
A solution is a homogeneous mixture of two or more substances, where the substances are evenly distributed throughout the mixture. To fully understand the nature of a solution, it’s essential to delve into its basic components: solute and solvent.
The Solute: The Guest
The solute is the substance being dissolved. It’s the guest molecule that enters the solvent’s space, creating a homogeneous mixture. Imagine a tea bag steeping in hot water. The tea leaves represent the solute, releasing their flavorful compounds into the water (solvent).
The Solvent: The Host
The solvent is the substance that dissolves the solute. It’s the host molecule that welcomes the solute into its molecular environment. In our tea example, water acts as the solvent, allowing the tea leaves to disperse and release their flavors.
The Interplay of Solute and Solvent
The solute and solvent interact through various forces, forming a stable solution. These forces can range from electrostatic interactions to hydrogen bonding, depending on the nature of the substances involved. The concentration of the solution refers to the amount of solute dissolved in a given amount of solvent.
Applications of Solutions: A Vast Toolkit
Solutions play a versatile role in our world, from medical applications to industrial processes and everyday life. Medical solutions like saline can be used for intravenous fluids or wound cleansing. Cleaning agents often contain solutions that dissolve dirt and grease. Even our food is a collection of solutions, including the water we drink and the sugary beverages we enjoy.
By understanding the components and interactions of solutions, we can appreciate their diverse applications and harness their power for various purposes. Whether it’s in the medical field, industry, or our daily lives, solutions are essential to our well-being and progress. Further exploration and research into solutions will continue to unlock their full potential and contribute to advancements in various fields.
Delving into the Enigmatic World of Solutions: Unraveling Their Key Components and Interactions
In the realm of chemistry, the concept of “solution” holds a pivotal role. It encompasses a vast array of substances encountered in our daily lives, from the salty tang of seawater to the refreshing sweetness of lemonade. To fully grasp the multifaceted nature of solutions, it is essential to delve into their fundamental components and explore how they interact to form these homogeneous mixtures.
Key Components of a Solution
The foundation of a solution lies in the interplay of two distinct substances: the solute and the solvent. The solute is the substance being dissolved, while the solvent is the medium in which it dissolves. Their intricate dance gives rise to a myriad of solutions with diverse properties and applications.
Solute: The Guest
Imagine the solute as a guest invited to a grand party. It is the substance that eagerly ventures into the solvent, seeking to mingle and become a part of the larger ensemble. The concentration of the solute, measured in units such as moles per liter, determines the extent of its presence in the solution, influencing its overall characteristics.
Solvent: The Host
The solvent, on the other hand, acts as a hospitable host, welcoming the solute into its embrace. Its role is to provide the solute with a conducive environment where it can fully dissolve and distribute evenly throughout the solution. The nature of the solvent, such as its polarity or acidity, plays a crucial role in determining the solubility and properties of the resulting solution.
Interplay of Solute and Solvent: A Harmonious Dance
The interaction between solute and solvent is a dance of molecular exchange and rearrangement. The solute particles disperse within the solvent molecules, breaking down into smaller units and forming new bonds. This delicate balance between solute-solvent interactions shapes the characteristics of the solution, influencing its physical and chemical properties.
Examples of Solutions: From Nature’s Embrace to Man-made Wonders
Solutions are ubiquitous in our world, gracing us with their presence in countless forms. Natural wonders such as saltwater oceans and Earth’s atmosphere exemplify the harmonious coexistence of solutes and solvents. Human ingenuity has also harnessed the power of solutions to create a multitude of products, including medical treatments, cleaning agents, and even the delectable flavors of our favorite beverages.
Applications of Solutions: Versatility Unbound
The versatility of solutions extends far beyond the realms of chemistry. They find indispensable applications in a wide spectrum of scientific disciplines, industries, and everyday life. From medical advancements to industrial processes and culinary artistry, solutions play an integral role in shaping our world.
Our exploration of the components and interactions within solutions has unveiled the intricate tapestry of these homogeneous mixtures. Understanding their key building blocks and how they orchestrate to form solutions is paramount for unraveling the mysteries of chemistry and appreciating the ubiquitous presence of solutions in our world. May this journey ignite your curiosity and inspire you to delve deeper into the fascinating realm of solutions.
Components of a Solution: Understanding the Building Blocks of Mixtures
Welcome to the world of solutions, where substances dance together to form new and mysterious mixtures. As we embark on this journey, it’s imperative to understand the fundamental components that make up these solutions. They hold the key to unlocking the secrets that lie within.
At the heart of a solution lies the solute, the substance that seeks refuge in the embrace of the solvent. The solute, like a shy guest, dissolves, disappearing into the larger presence of the solvent. This harmonious union creates a new entity characterized by its concentration, a delicate balance that determines the strength of the solution.
The interplay between solute and solvent is a dance of give and take. The solute surrenders its individuality, seeping into the solvent’s embrace, while the solvent welcomes this transformation, expanding its boundaries to accommodate its new companion. As this dynamic duo twirls, they give birth to a solution with unique properties, distinct from either of its original components.
Importance of Understanding Solution Components
Unveiling the secrets of solutions is not just an academic pursuit; it’s a window into the world around us. From the salty tang of the ocean to the sweet embrace of a sugary treat, solutions shape our daily lives.
In the hospital, solutions carry life-saving medicines to our bodies, fighting off diseases and healing wounds. In the laboratory, solutions provide a canvas for scientific experiments, unveiling the wonders of chemistry. Even in our kitchens, solutions play a pivotal role, transforming ingredients into culinary masterpieces.
Applications of Solutions: Endless Possibilities
Solutions touch every corner of our existence, from the clothes we wear to the air we breathe. They are the backbone of countless industries, offering solutions to a myriad of challenges.
In agriculture, solutions bring nourishment to crops, boosting yields and feeding growing populations. In industry, solutions provide the foundation for everything from plastics to pharmaceuticals, shaping the modern world. Even in our homes, solutions clean, disinfect, and protect our living spaces.
As we conclude our exploration into the components of solutions, let us remember their profound importance. By understanding these building blocks, we unlock a world of possibilities. They provide a gateway to innovation, better health, and a deeper appreciation for the wonders of our surroundings.
So, dear reader, let us continue our journey, delving into the fascinating depths of solutions, uncovering the secrets they hold and unlocking their limitless potential.
Encouragement for further exploration and research.
Unveiling the Building Blocks of a Solution: A Journey into Substance Mixtures
Delving into the realm of chemistry, we encounter a fascinating concept known as a solution. A solution is essentially a homogeneous mixture of two or more substances that have merged seamlessly to create a new entity. Understanding the components of a solution is crucial for comprehending the behavior and properties of these remarkable substances.
The Solute: The Essence Dissolved
Within a solution, one substance takes on the role of the solute, the substance that dissolves into another substance, known as the solvent. The solute is the very essence of the solution, giving it unique characteristics. The amount of solute present in a solvent determines the concentration of the solution, a crucial factor in shaping its properties.
The Solvent: The Dissolver’s Domain
The solvent, on the other hand, is the accommodating substance that provides a home for the solute. It is the dominant component, responsible for establishing the solution’s physical state and many of its properties. The interplay between the solute and the solvent is a delicate dance, one that can lead to a variety of solution types, from dilute to concentrated.
The Interplay of Solute and Solvent: A Dynamic Duet
The interaction between solute and solvent is a fascinating spectacle. As the solute enters the solvent, it begins a journey of dispersion, breaking down into individual particles that mingle seamlessly with the solvent molecules. This process, known as solvation, creates a homogeneous mixture where the solute is evenly distributed throughout the solvent. The concentration of the solution directly influences the extent of solvation and, consequently, the properties of the solution.
Real-World Encounters with Solutions: From the Sea to the Stars
Solutions are not confined to the realm of chemistry labs; they permeate our everyday lives. From the salty depths of the ocean to the gaseous expanse of the atmosphere, solutions play a pivotal role. Salt water, a familiar solution, is a mixture of salt dissolved in water, while air, essential for life, is a solution of gases, primarily nitrogen and oxygen.
Harnessing the Power of Solutions: A Multifaceted Application
The applications of solutions are as diverse as the solutions themselves. Medical solutions, for instance, bring relief to ailments and heal wounds. Cleaning agents, often solutions of detergents or acids, banish dirt and grime. Even the food we consume contains solutions, such as juices, soups, and sauces.
Embarking on a Journey of Discovery: Further Exploration
Understanding the components of a solution is merely a gateway to a world of fascinating chemistry. Further exploration beckons, inviting us to delve deeper into the intricate interactions between solutes and solvents, to uncover the secrets of different solution types, and to witness the myriad applications of these remarkable substances. The journey of discovery awaits, promising insights into the fundamental building blocks of our world.