Absolute Zero: The Ultimate Guide To Understanding And Converting Temperatures
Absolute zero, the hypothetical lower limit of temperature, marks the point where thermal energy completely vanishes and molecular movement ceases. Represented as 0 K on the Kelvin scale, it corresponds to -273.15 °C on the Celsius scale. This point allows for the study of ultra-low temperatures in cryogenics and phenomena like superconductivity. Understanding conversion formulas is crucial for accurate temperature interpretation, such as converting between Celsius and Fahrenheit. Absolute zero provides a fundamental reference point for understanding thermal energy and molecular motion, as it represents the hypothetical state where these properties cease to exist.
Unraveling the Enigma of Absolute Zero
In the realm of temperature, there exists a profound concept that challenges the limits of our understanding: absolute zero. This elusive threshold marks the theoretical lower limit of temperature, where all thermal energy is stripped away, and molecular motion ceases.
Represented as 0 degrees Kelvin (K), absolute zero is a fundamental reference point in thermodynamics. It signifies a state of absolute coldness, where the absence of thermal energy renders matter devoid of all thermal activity.
The concept of absolute zero has captivated scientists for centuries, fueling advancements in various fields such as cryogenics, where the study of ultra-low temperatures unlocks secrets of materials and their behavior. At or near absolute zero, exotic phenomena like superconductivity emerge, defying conventional understanding of electrical properties.
**The Interplay of Temperature Scales: Unraveling the Enigma of Absolute Zero**
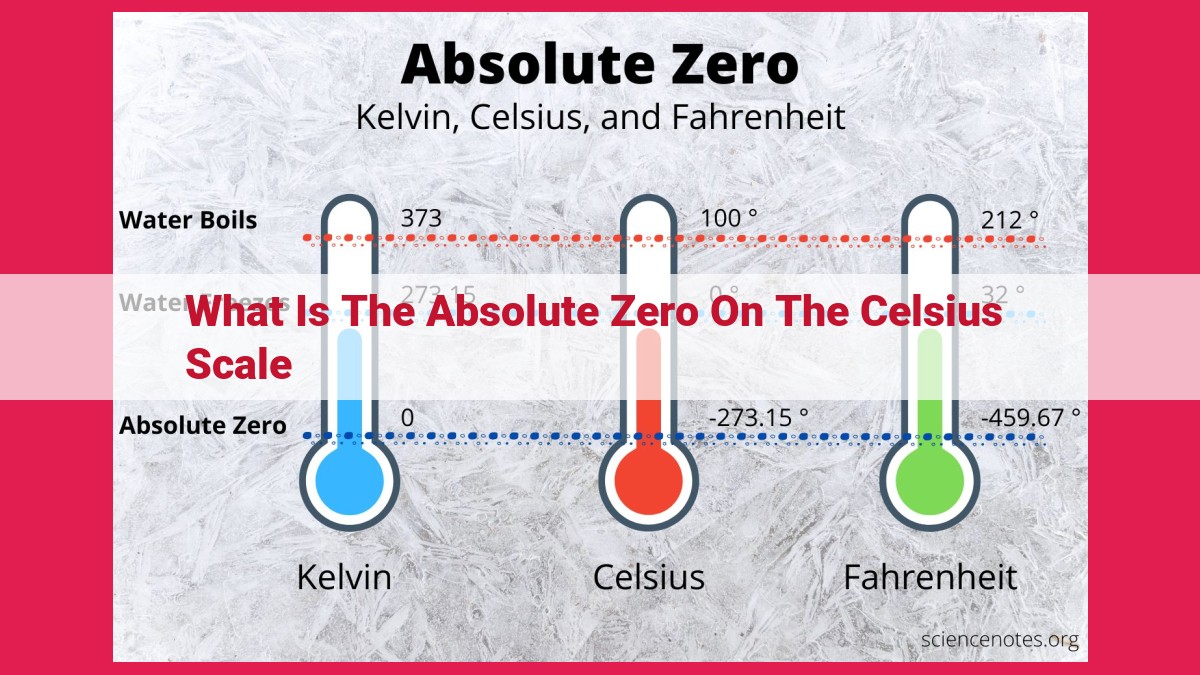
Temperature, an elusive concept that governs the motion of molecules and dictates the behavior of matter, is measured using various scales. Two of the most commonly used scales are Celsius and Kelvin. While Celsius assigns 0°C to the freezing point of water and 100°C to its boiling point, Kelvin takes a more fundamental approach by aligning its zero point with absolute zero.
Absolute zero, represented as 0 Kelvin (K), marks the theoretical lower limit of temperature at which all thermal energy is *absent*. This enigmatic state signifies the cessation of molecular motion, a realm where matter exhibits extraordinary properties. In contrast to the Celsius scale, absolute zero corresponds to -273.15°C, emphasizing the profound difference in their conceptual foundations.
The interplay between these temperature scales is crucial for understanding thermodynamic phenomena. Converting between Celsius and Kelvin is essential for precise temperature interpretation. The formula, K = °C + 273.15, facilitates this conversion, allowing scientists to seamlessly navigate between the two scales.
For example, the freezing point of water, expressed as 0°C on the Celsius scale, translates to 273.15 K on the Kelvin scale. Conversely, absolute zero, designated as 0 K, equates to -273.15°C on the Celsius scale. This intricate relationship highlights the fundamental distinction between the two scales.
Comprehending the interplay of temperature scales provides a deeper appreciation of absolute zero and its implications in thermodynamics. It empowers us to decipher the hidden language of temperature, unlocking the secrets of molecular behavior and the enigmatic realm of ultra-low temperatures.
Understanding Absolute Zero: A Journey Through Thermodynamics
Absolute Zero: The Frigid Limit
In the realm of thermodynamics, the notion of absolute zero reigns supreme as the theoretical lower limit of temperature. This elusive point marks the point where molecular motion ceases and thermal energy vanishes. Absolute zero is denoted as 0 Kelvin (K) in the Kelvin scale, which scientists widely regard as the most fundamental temperature scale.
Temperature Scales: A Comparative Perspective
Various temperature scales exist, including Celsius and Kelvin. The Celsius scale uses the freezing point of water as its zero point, while the Kelvin scale aligns its zero point with absolute zero. This alignment makes the Kelvin scale the most scientifically accurate and widely used scale in thermodynamics.
Cryogenics: Delving into Ultra-Low Temperatures
The pursuit of ultra-low temperatures has led to the development of cryogenics, a fascinating field that explores the behavior of matter at these extreme conditions. Cryogenic techniques find applications in various fields, such as preserving biological materials and advancing medical treatments.
Superconductivity: A Quantum Dance at Absolute Zero
At or near absolute zero, a remarkable phenomenon called superconductivity emerges. In this state, materials exhibit zero electrical resistance, allowing electricity to flow without any loss. Superconductivity holds immense potential for future technologies, including energy-efficient power transmission and ultra-fast computing.
The Fahrenheit Scale: A Common Household Tool
Despite the scientific superiority of the Kelvin scale, the Fahrenheit scale remains widely used in everyday applications. Its zero point aligns with the freezing point of a mixture of water and salt, making it convenient for household purposes.
Absolute zero serves as a cornerstone in thermodynamics, providing a profound understanding of the behavior of matter at extremely low temperatures. From cryogenics to superconductivity, exploring related concepts enhances our grasp of this intriguing phenomenon. By embracing the insights gained from absolute zero, we unlock the potential to unravel further mysteries and advance scientific discoveries in the years to come.
Temperature Conversion: Understanding the Interplay of Scales
In the realm of understanding temperature, it’s crucial to master the art of temperature conversion for accurate interpretation. The conversion formulas serve as the bridge between different temperature scales, enabling us to communicate and compare temperatures seamlessly.
One of the most common conversions involves Celsius and Fahrenheit, two widely used scales. To convert from Celsius to Fahrenheit, we employ the formula:
°F = (°C x 1.8) + 32
For instance, to convert 20°C to Fahrenheit, we have:
°F = (20 x 1.8) + 32
°F = 36 + 32
°F = 68
Therefore, 20°C corresponds to 68°F.
Conversely, to convert from Fahrenheit to Celsius, we utilize the formula:
°C = (°F - 32) / 1.8
Taking 86°F as an example:
°C = (86 - 32) / 1.8
°C = 54 / 1.8
°C = 30
So, 86°F translates to 30°C.
Understanding these conversions is essential in various fields, from meteorology to food preparation. By harnessing the power of conversion formulas, we can navigate the temperature landscape with ease and communicate our findings with precision.
Thermal Energy and Molecular Motion: Absolute Zero’s Impact
The Essence of Thermal Energy
Thermal energy is an intriguing concept that describes the kinetic energy of molecules within a substance. Imagine a bustling dance floor, where each molecule is a dancer, moving in countless directions and colliding with one another. This collective movement, known as molecular motion, gives rise to the substance’s temperature.
Absolute Zero: A Profound State
At the other end of the temperature spectrum lies *absolute zero*, the theoretical lower limit where all molecular motion ceases. It’s like a cosmic ballet where the dancers have frozen in place, motionless and silent. At this enigmatic point, thermal energy vanishes entirely.
A Silent Symphony
Absolute zero represents a complete absence of thermal energy. The molecules, once so energetic, have now lost their momentum. It’s a state of perfect stillness, where the symphony of molecular motion has been muted.
Zero Point on the Kelvin Scale: An Absolute Alignment
In the realm of physics, the Kelvin scale stands out as a prominent temperature scale that takes absolute zero as its reference point. This zero point, denoted as 0 K, aligns precisely with the theoretical lower limit of temperature, a point where the laws of thermodynamics dictate a complete absence of thermal energy.
The significance of this alignment lies in its implications for molecular behavior. As temperature approaches absolute zero, molecular motion slows down to a virtual standstill. This cessation of motion indicates the absence of thermal energy, leaving molecules in their lowest energy state. The zero point on the Kelvin scale thus represents a state of absolute molecular stillness, a fascinating phenomenon that challenges our intuition about the nature of matter.
This alignment between absolute zero and the zero point on the Kelvin scale serves as a cornerstone of thermodynamics, enabling scientists to accurately measure and quantify temperatures across a wide range of physical systems. By understanding the significance of zero point, researchers gain a deeper understanding of the behavior of molecules and the fundamental principles of thermal energy.