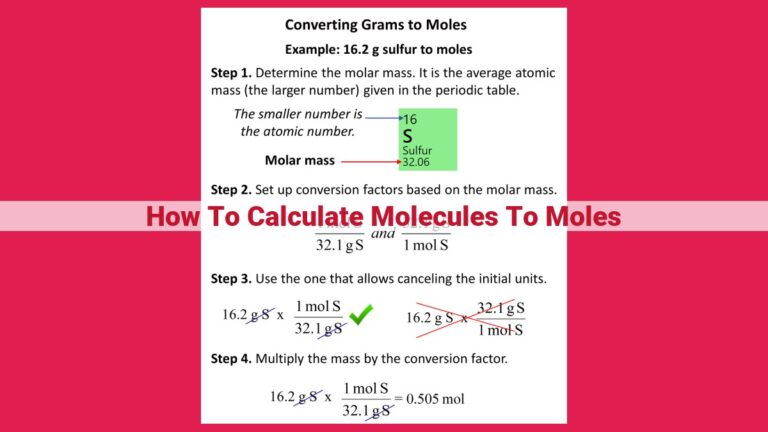
Unlocking The Secrets Of Moles: A Comprehensive Guide To Molarity And Beyond
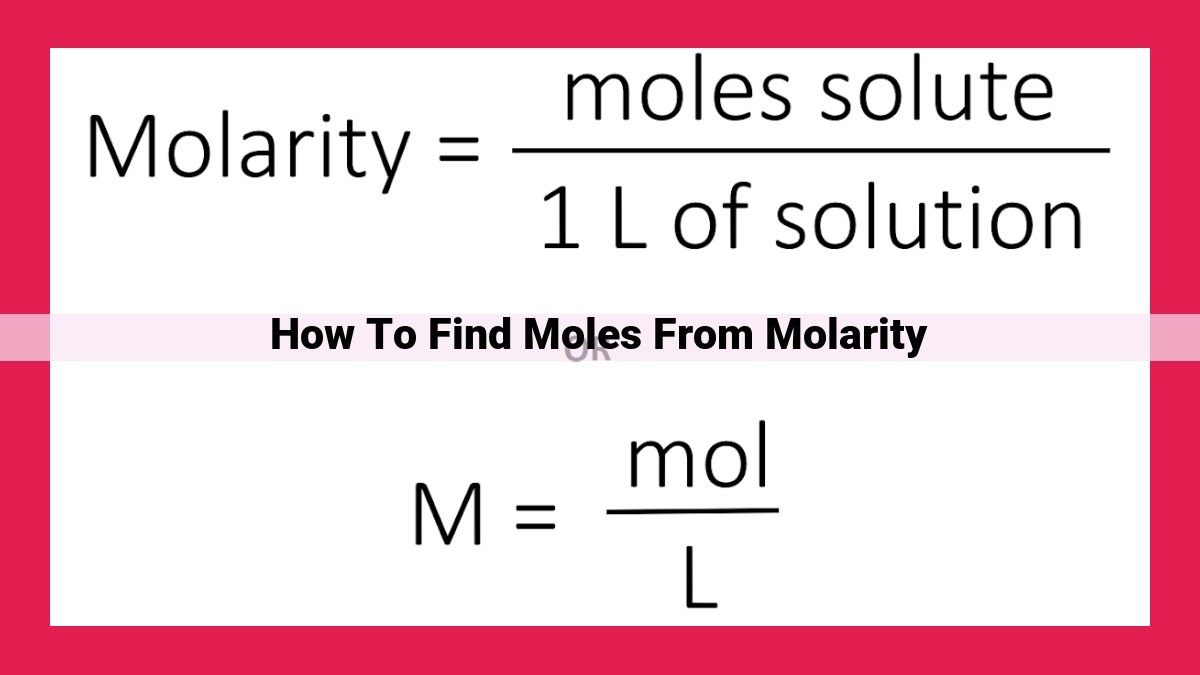
Finding moles from molarity is crucial in chemistry to determine the amount of substance present in a solution. Molarity, volume, and molecular weight are interlinked concepts. The formula n = M x V / MW calculates moles directly. Accurate understanding of these concepts ensures precise determination of moles. It finds applications in mass-molarity-volume relationships, percentage concentration, density, and specific gravity calculations.
Finding Moles from Molarity: A Chemistry Concept Unveiled
In the realm of chemistry, measuring the concentration of solutions is crucial for a myriad of reactions and experiments. Understanding how to find moles from molarity is a fundamental concept that unlocks the door to comprehending and quantifying chemical substances. Whether you’re a seasoned chemist or a budding enthusiast, this guide will unravel the intricacies of this essential calculation, illuminating its importance as a cornerstone of chemistry.
Key Concepts Demystified:
Before delving into the intricacies of the formula, it’s imperative to delve into some key concepts that lay the groundwork for understanding moles and molarity.
-
Molarity: A measure of concentration expressed in moles of solute per liter of solution. It provides a quantitative insight into the abundance of a specific substance within a given volume.
-
Volume: A measurement of the space occupied by a substance, typically expressed in liters (L), milliliters (mL), or cubic centimeters (cm³). Accurate volume measurements are essential for precise calculations involving molarity.
-
Moles: The standard unit for measuring the amount of a substance, representing the quantity of atoms, molecules, or ions present. Avogadro’s number (6.022 x 10^23) serves as the conversion factor between moles and individual particles.
-
Molecular Weight: A measure of the mass of one mole of a compound or substance. It essentially represents the sum of the atomic weights of all the atoms in a molecule.
Calculating Moles from Molarity:
Now that we’ve established a solid foundation, let’s delve into the formula that empowers us to find moles from molarity:
n = M x V / MW
Where:
- n represents the number of moles
- M represents the molarity in moles per liter (M)
- V represents the volume of the solution in liters (L)
- MW represents the molecular weight of the solute in grams per mole (g/mol)
This equation embodies the interplay between moles, molarity, volume, and molecular weight. By manipulating these variables, we can accurately determine the number of moles present in a given solution.
Interrelationships and Significance:
The relationship between moles, molarity, volume, and molecular weight is directly proportional. An increase in any of these factors will result in an increase in the number of moles. Conversely, a decrease in these variables will lead to a decrease in the number of moles.
Understanding these interrelationships is paramount for conducting accurate chemical experiments, making informed decisions, and drawing meaningful conclusions from experimental data. By mastering this concept, you unlock the ability to quantify the concentration of solutions, perform stoichiometric calculations, and delve deeper into the fascinating world of chemistry.
Stay tuned for the upcoming sections, where we’ll explore other related concepts, practical applications, and provide additional resources to enhance your understanding of finding moles from molarity.
Understanding Key Concepts:
- Molarity: Explanation of molarity as a measure of concentration and its units (M).
- Volume: Importance of volume measurement and its common units (L, mL, cm³).
- Moles: Definition of moles as a unit of amount of substance and Avogadro’s number.
- Molecular Weight: Significance of molecular weight in determining the mass of one mole of a substance.
Understanding Key Concepts: The Essentials for Finding Moles from Molarity
In the realm of chemistry, understanding the relationship between moles, molarity, volume, and molecular weight is crucial. These concepts are the building blocks for accurately determining the amount of substance present in a solution.
Molarity: A Measure of Concentration
Molarity measures the concentration of a solution, indicating the number of moles of solute (the substance being dissolved) per liter (L) of solution. It is represented by the symbol M and expresses the amount of substance in a volume of solution.
Volume: The Space Occupied
Volume measures the amount of space occupied by a substance. It is usually expressed in liters (L), milliliters (mL), or cubic centimeters (cm³). Accurately measuring volume is essential for determining the number of moles present in a solution.
Moles: A Unit of Amount
Moles represent the amount of a substance present in a sample. It is defined as the amount of substance that contains as many elementary entities (atoms, molecules, ions) as there are atoms in 0.012 kilograms of carbon-12. This number is known as Avogadro’s number and is approximately 6.022 x 10^23.
Molecular Weight: Determining Mass of One Mole
Molecular weight represents the mass of one mole of a substance. It is the sum of the atomic masses of all the constituent atoms in the molecule. Understanding molecular weight helps determine the mass associated with one mole of a substance.
By understanding these key concepts, we lay the foundation for accurately calculating moles from molarity, enabling us to determine the amount of substance present in a solution, a fundamental skill in the world of chemistry.
Calculating Moles from Molarity: A Chemical Odyssey
In the realm of chemistry, understanding the intricate relationship between molarity, volume, moles, and molecular weight is paramount to unraveling the mysteries of solutions. This knowledge empowers us to determine the exact number of moles present in a given solution, a crucial step in countless chemical calculations.
The formula for calculating moles from molarity is a guiding compass in this endeavor:
n = M x V / MW
Where:
- n represents the number of moles (the unit amount of substance)
- M denotes the molarity (the concentration of the solution in moles per liter)
- V signifies the volume of the solution (in liters)
- MW stands for the molecular weight (the mass of one mole of the substance, in grams per mole)
Imagine yourself as a chemist tasked with preparing a specific volume of a solution with a precise concentration. Armed with the formula above, you can embark on this mission with confidence. By carefully measuring the volume of the solution and determining its molarity, you can then calculate the exact number of moles required.
This knowledge is not merely an academic exercise; it finds practical applications in various fields. For instance, in analytical chemistry, determining the number of moles present in a solution is essential for conducting accurate titrations and quantifying the concentration of unknown substances. In pharmaceutical chemistry, it guides the precise preparation of drug solutions with the correct dosage.
Furthermore, understanding the relationship between moles, molarity, volume, and molecular weight provides a solid foundation for exploring other related concepts in chemistry, such as the mass-molarity-volume relationship and percentage concentration. This comprehensive understanding empowers chemists to navigate the intricacies of solution chemistry with ease.
In conclusion, the ability to calculate moles from molarity is a fundamental skill for any chemist. It allows us to decipher the composition of solutions, prepare precise reagents, and delve deeper into the fascinating world of chemical reactions. As you embark on your journey in chemistry, remember the formula n = M x V / MW as your faithful companion, guiding you towards a profound understanding of solutions and their boundless applications.
Understanding the Interrelationship of Moles, Molarity, Volume, and Molecular Weight
In chemistry, understanding the relationship between moles, molarity, volume, and molecular weight is crucial for accurately determining the amount of substance present in a solution. These concepts are intertwined, and a clear comprehension of each is essential for a deep understanding of chemistry.
Molarity, expressed in moles per liter (M), measures the concentration of a solution. It represents the number of moles of solute dissolved in one liter of solvent. Volume quantifies the space occupied by a substance, typically measured in liters (L), milliliters (mL), or cubic centimeters (cm³).
Moles represent the amount of substance, defined as the number of elementary entities (atoms, molecules, or ions) present in a given sample. Avogadro’s number (6.022 x 10²³) relates the number of entities to the number of moles.
Molecular weight indicates the mass of one mole of a substance, expressed in grams per mole (g/mol). It is crucial for determining the mass of a given number of moles.
The formula that connects these concepts is n = M x V / MW, where:
- n is the number of moles
- M is the molarity
- V is the volume
- MW is the molecular weight
This formula enables the calculation of moles from the known values of molarity, volume, and molecular weight. The interrelationship of these concepts highlights the direct proportionality between moles, molarity, volume, and molecular weight. Understanding this relationship is vital for accurate determination of the number of moles present in a solution, a fundamental step in various chemical applications.
Understanding the Correlation: Finding Moles from Molarity
In the realm of chemistry, comprehending the relationship between moles, molarity, volume, and molecular weight is crucial. This intricate interplay allows us to determine the number of moles present in a solution, a concept of utmost importance in various chemical endeavors.
Bridging the Concepts:
Just as a recipe requires precise measurements of ingredients, determining the moles of a substance necessitates understanding these key concepts:
- Molarity (M): A measure of concentration, expressing the amount of moles of a solute dissolved in liters of solution.
- Volume (L): The space occupied by the solution, measured in units such as liters, milliliters, or cubic centimeters.
- Moles (mol): A unit representing the amount of substance, equivalent to Avogadro’s number (6.022 x 10^23).
- Molecular Weight (g/mol): The combined mass of all atoms in one molecule of a substance, expressed in grams per mole.
Unveiling the Formula:
To calculate the moles (n) in a solution, we employ the formula:
n = M x V / MW
Where:
- M is the molarity in moles per liter
- V is the volume in liters
- MW is the molecular weight in grams per mole
This formula unveils the direct relationship between these quantities. By understanding their interdependence, we can accurately determine the number of moles present in a solution.
Expanding Our Horizons:
Beyond these core concepts, several other related concepts broaden our understanding of solution concentration:
- Mass-Molarity-Volume Relationship: This equation links the mass of a solute, its molarity, and the solution’s volume.
- Percentage Concentration: An alternative method of expressing solution concentration, representing the solute’s presence as a percentage of the solution’s mass or volume.
- Density and Specific Gravity: These concepts measure the mass per unit volume and relative density of substances, providing additional insights into concentration.
Practical Significance:
The ability to find moles from molarity finds widespread application in chemistry and related fields, including:
- Stoichiometric Calculations: Balancing chemical equations and determining reactant and product quantities.
- Solution Preparation: Dissolving specific amounts of solutes to achieve desired concentrations.
- Titrations: Determining the concentration of unknown solutions by reacting them with known solutions.
- Electrochemistry: Understanding the behavior of ions in solution and their role in electrochemical reactions.
By mastering these concepts, we unlock a gateway to deeper understanding in chemistry and empower ourselves to tackle a vast array of challenges with confidence.