Tropomyosin: A Pivotal Protein In Skeletal Muscle Contraction And Plasticity
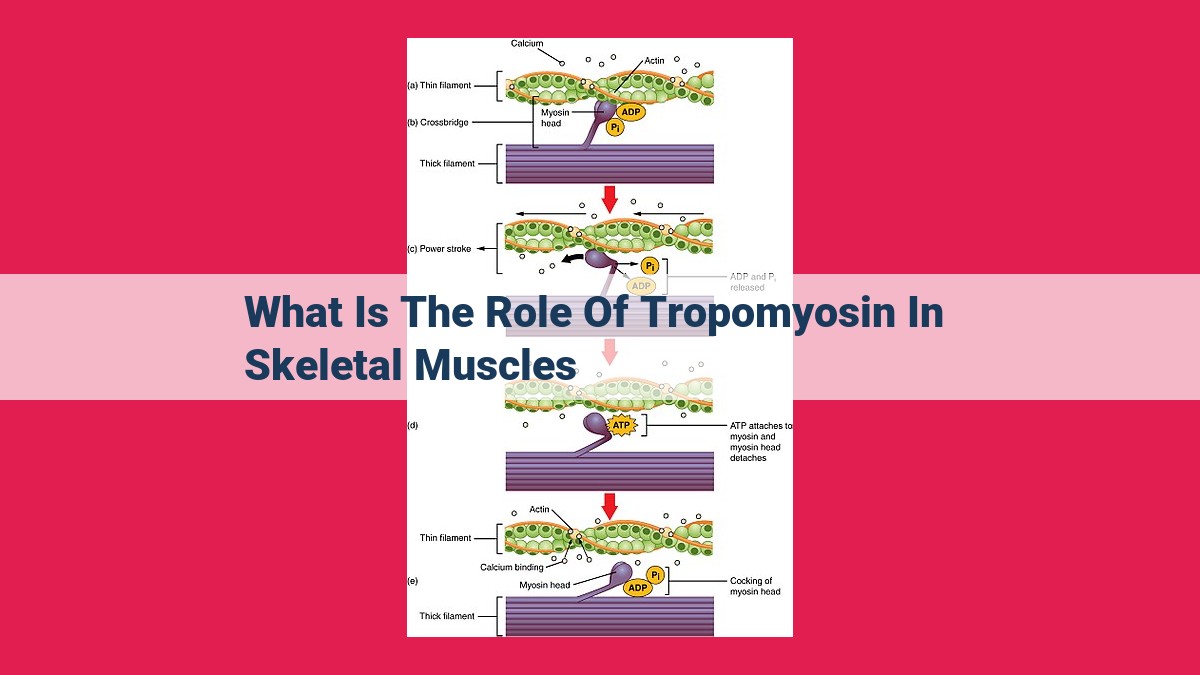
Tropomyosin, a thin filament protein in skeletal muscles, plays a pivotal role in muscle contraction. By sliding past myosin filaments, tropomyosin facilitates the interaction between actin and myosin, allowing for muscle shortening. Its binding to the troponin complex regulates the availability of myosin binding sites on actin. When calcium ions trigger a conformational change in tropomyosin, it uncovers these sites, initiating muscle contraction. Tropomyosin is crucial for maintaining muscle homeostasis and plasticity, ensuring efficient muscle function and adaptation to various stimuli.
Unraveling the Secrets of Tropomyosin: A Journey into Skeletal Muscle
In the intricate world of skeletal muscles, a remarkable protein named tropomyosin plays a pivotal role in the captivating ballet of muscle contraction. Let’s embark on an exciting exploration to discover its location within the muscle machinery and unravel its critical function in the dance of movement.
Tropomyosin’s Abode: The Sarcomere
Muscles are an intricate web of sarcomeres, the fundamental building blocks of their contractile apparatus. Within each sarcomere, a delicate network of proteins exists, with actin and myosin taking center stage. Tropomyosin, a thin filament protein, makes its home within this molecular ensemble, residing along the actin filaments. It’s like the scaffolding that holds up the actin, providing a platform for the muscular performance that’s about to unfold.
Unveiling Tropomyosin’s Vital Role in Muscle Contraction
The magic of muscle contraction lies in the intricate interplay between actin and myosin. Tropomyosin acts as a gatekeeper, regulating the interaction between these two dance partners. When the muscle is at rest, tropomyosin blocks the binding sites on actin, preventing myosin from attaching. But when the muscle receives a signal to contract, things change in an instant. Calcium ions, like tiny messengers, are released from the muscle’s storage chambers, the sarcoplasmic reticulum. These ions bind to another protein, troponin, which is closely associated with tropomyosin.
As troponin changes its shape, it pulls tropomyosin along with it, uncovering the binding sites on actin. This is the turning point, the cue for myosin to step up and form cross-bridges with actin. The dance of contraction begins. Myosin flexes its molecular muscles, pulling the actin filaments towards the center of the sarcomere, shortening the muscle fibers and generating movement.
Tropomyosin’s Symphony with Troponin: A Coordinated Effort
Tropomyosin’s tango with troponin is a delicate balancing act. Together, they form a regulatory complex that orchestrates the timing and coordination of muscle contraction. Troponin, like a sensitive switch, responds to the slightest changes in calcium concentration, triggering the conformational shift in tropomyosin that opens the door for myosin’s engagement. This intricate interplay ensures that muscle contraction occurs only when it’s intended, providing precise control over movement.
Tropomyosin, the unsung hero of skeletal muscles, plays a central role in the captivating choreography of muscle contraction. Its location on the thin filaments, its interaction with troponin, and its response to calcium ions make it an essential player in orchestrating the symphony of movement. By understanding tropomyosin’s vital function, we gain a deeper appreciation for the complex and elegant machinery that animates our bodies.
Unveiling Tropomyosin’s Function in Muscle Contraction:
- Explain the sliding filament theory and tropomyosin’s role in facilitating the interaction between actin and myosin.
Unveiling Tropomyosin’s Crucial Role in Muscle Contraction
In the realm of skeletal muscles, a remarkable symphony of molecular events unfolds during contraction. One of the key players in this intricate dance is a protein known as tropomyosin. Journey with us as we delve into tropomyosin’s pivotal role in muscle contraction, a process that powers every movement we make.
The Sliding Filament Theory
Picture your skeletal muscle as a bundle of tiny thread-like structures called myofibrils. These myofibrils, in turn, are composed of smaller units known as sarcomeres. Within each sarcomere, two types of filaments- actin and myosin– slide past each other, like microscopic trains on parallel tracks.
Tropomyosin’s Role
This is where tropomyosin steps into the spotlight. It’s a thin, filamentous protein that lies along the actin filaments, acting as a gatekeeper that prevents myosin from binding to actin. Without tropomyosin’s presence, the filaments would be constantly stuck together, rendering muscle contraction impossible.
The Calcium Trigger
But what flips the switch for muscle contraction? Enter calcium ions. When a nerve impulse reaches a muscle, it triggers the release of calcium from the sarcoplasmic reticulum, a specialized compartment within the muscle cell. This sudden influx of calcium prompts a conformational change in tropomyosin, causing it to shift its position and expose the binding site on actin.
Myosin’s Embrace
With tropomyosin out of the way, myosin molecules can finally reach out and bind to actin. This interaction initiates the power stroke, the fundamental movement that drives muscle contraction. Myosin pulls on actin, causing the filaments to slide past each other and shortening the sarcomere.
The Beat Goes On
This intricate ballet continues until the nerve impulse ceases and calcium levels return to normal. Tropomyosin then swings back into place, blocking the binding site on actin and halting muscle contraction.
Tropomyosin’s Importance
Tropomyosin’s role is not just confined to muscle contraction; it also contributes to muscle homeostasis by preventing uncontrolled actin-myosin interactions. Additionally, tropomyosin isoforms, variations in its protein structure, are thought to play a crucial role in muscle plasticity, the muscle’s ability to adapt to different functional demands.
In conclusion, tropomyosin is an essential player in the intricate symphony of muscle contraction. By regulating actin-myosin interactions, it ensures that our bodies move with purpose and precision, transforming the marvels of molecular mechanics into the expressive power of human movement.
Exploring Tropomyosin’s Interaction with Troponin: A Molecular Dance in Muscle Contraction
In the intricate world of muscle contraction, the interaction between tropomyosin and troponin plays a crucial role. Tropomyosin, a thin filament protein, forms a helical structure that lies along the groove of the actin filament. Like a guardian, it regulates muscle contraction by controlling the interaction of actin with another key player, myosin.
Enter troponin, a protein complex consisting of three subunits: troponin I, troponin T, and troponin C. Troponin I binds to actin, troponin T to tropomyosin, and troponin C to calcium ions. This complex acts as a gatekeeper, modulating the interaction between tropomyosin and actin.
In the absence of calcium ions, troponin and tropomyosin together block the myosin-binding site on actin. It’s like a double lock, preventing muscle contraction. However, when calcium ions flood the muscle during an action potential, they bind to troponin C. This binding triggers a conformational change in troponin, which in turn causes a shift in tropomyosin.
The movement of tropomyosin uncovers the myosin-binding site on actin, allowing myosin to bind and initiate the sliding filament mechanism. This molecular dance gives rise to muscle contraction, the force that powers our every movement.
Without this intricate interaction between tropomyosin and troponin, muscle contraction would be impossible. These proteins are the gatekeepers of muscle function, ensuring that our bodies can move gracefully and perform the tasks that make up our lives.
**Calcium Ions: Unlocking the Secrets of Tropomyosin Regulation**
Deep within the intricate realm of our muscles, a protein called tropomyosin orchestrates the delicate dance of muscle contraction. Its function, regulated by the calcium ions released from the sarcoplasmic reticulum, provides a fascinating glimpse into the intricate machinery of human movement.
Calcium ions act as molecular messengers, triggering a conformational change in tropomyosin that ultimately leads to muscle contraction. When calcium is present, it binds to troponin C, a protein associated with tropomyosin. This binding event initiates a cascade of conformational changes that shift tropomyosin away from its inhibitory position.
As tropomyosin moves, it uncovers myosin-binding sites on the actin filament, enabling myosin to bind and interact with actin. This interaction fuels the sliding filament theory, where myosin filaments slide past actin filaments, generating the force that powers muscle contraction. The release of calcium ions effectively “unlocks” muscle contraction, allowing these filaments to engage in their coordinated dance.
Optimization for SEO on Page
- Keywords: Calcium ions, Tropomyosin, Muscle Contraction
- H2 Tag: Calcium Ions: Unlocking Tropomyosin Regulation
- Subheading: The Role of Calcium Ions in Tropomyosin Regulation
- Header 3: The Mechanics of Tropomyosin’s Conformational Change
Tropomyosin’s Significance in Muscle Physiology: A Symphony of Movement
Tropomyosin, an unsung hero within the skeletal muscle, plays a pivotal role in orchestrating the delicate balance and rhythmic contractions that power our every move. This thin filament protein elegantly resides within the sarcomere, the basic unit of muscle contraction, where it serves as a maestro, conducting the dance between actin and myosin, the essential contractile elements.
Tropomyosin’s exquisite choreography unfolds as calcium ions, released from the cellular depths, trigger a pivotal conformational change. This transformation exposes myosin-binding sites on actin, inviting myosin heads to engage in a harmonious dance. The interaction between these two dance partners propels the sliding movement of actin and myosin filaments, generating the force that propels our muscles.
Beyond its role in initiating contraction, tropomyosin also plays a crucial role in maintaining muscle homeostasis. It provides structural stability to the thin filaments, preventing unwanted filament collapse and ensuring efficient force generation. Furthermore, tropomyosin’s interaction with troponin, another regulatory protein, provides a fail-safe mechanism, preventing involuntary muscle contractions by modulating calcium sensitivity.
Tropomyosin’s significance extends beyond its involvement in muscle contraction. It also contributes to muscle plasticity, the ability of muscles to adapt to changing demands. By altering its expression levels or isoforms, muscles can fine-tune their contractile properties, ensuring optimal performance for specific tasks.
In conclusion, tropomyosin is not merely a passive player in muscle function. It is a dynamic regulator, ensuring precise and efficient muscle contractions, maintaining muscle homeostasis, and facilitating muscle plasticity. Without this unsung hero, our muscles would be paralyzed, unable to perform the intricate movements that define our existence.