Triple Point Of Water: A Foundational Benchmark For Temperature And Pressure Measurements
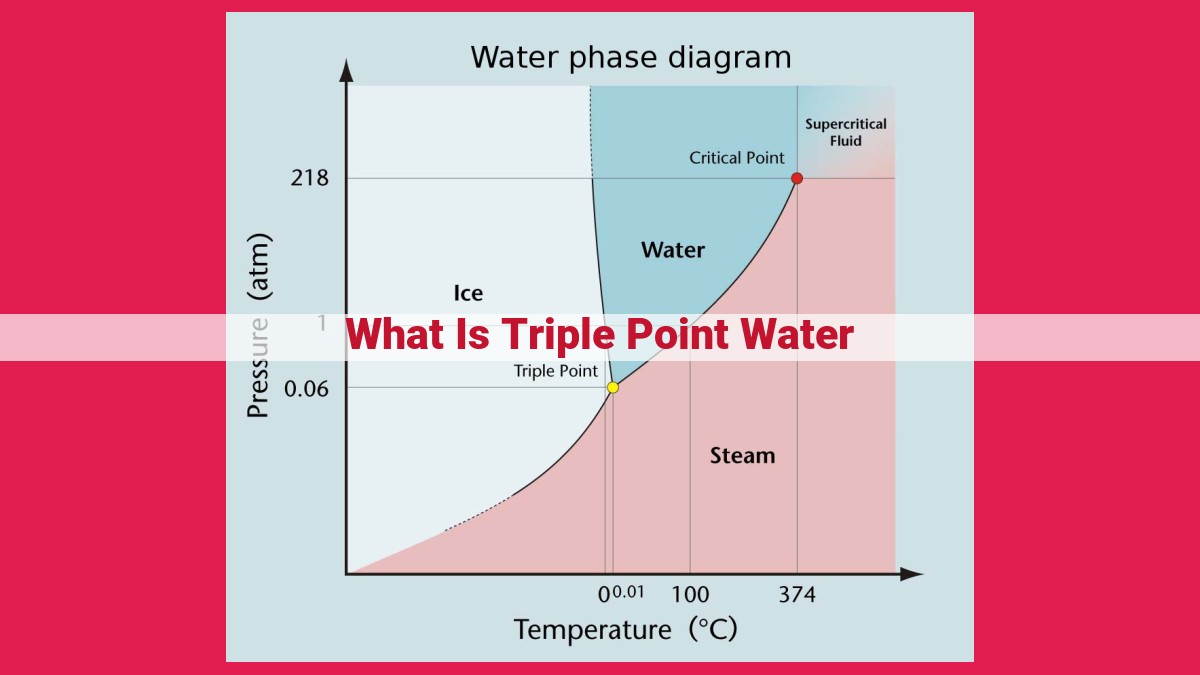
Triple point water is the unique temperature and pressure where all three phases of water (solid, liquid, and gas) coexist in equilibrium. It occurs at 0.01 degrees Celsius and 611.657 pascals. This point is significant as it provides a fixed reference for calibrating thermometers, defining the kelvin unit of temperature, and measuring pressure accurately. The triple point of water underpins various scientific and industrial applications, ensuring precise temperature and pressure readings for scientific research, temperature control systems, and pressure standards.
Understanding the Triple Point of Matter
In the world of physics and chemistry, the concept of the triple point holds immense significance. It marks the unique temperature and pressure at which a substance can exist in all three phases: solid, liquid, and gas, simultaneously. This phenomenon is not only fascinating but also has profound implications for our understanding of matter and its properties.
Phase Equilibrium and Phase Diagrams
Every substance has a specific phase diagram, a graphical representation that depicts the conditions under which it can exist in different phases. The triple point is the meeting point of three lines on a phase diagram, representing the three phases and their coexistence. At this point, the substance is in phase equilibrium, meaning that the rates of phase transitions are equal in both directions.
The triple point is a crucial concept in understanding phase transitions, the processes by which substances change from one phase to another. It provides insights into the delicate balance between the forces that hold molecules together in different states, allowing us to predict and control phase transitions in various applications.
The Triple Point of Water: A Story of Coexistence and Significance
As we venture into the fascinating world of matter, its properties, and phase transitions, the triple point of water emerges as a captivating phenomenon. It is a unique point where the solid, liquid, and gas phases of water coexist in perfect equilibrium.
At this precise temperature of 0.01°C and pressure of 611.73 pascals (or 0.006 atmospheres), water exhibits a remarkable dance of phases. Ice suspends in liquid water, while water vapor gracefully rises above. This delicate balance is a testament to the intricate interplay of energy and molecular interactions.
The triple point of water holds profound significance for understanding water’s thermodynamic behavior. It serves as a fixed reference point for temperature measurement, ensuring accuracy in scientific research and industrial applications alike. Moreover, it defines the Kelvin (K), the base unit of temperature in the International System of Units (SI).
Furthermore, the triple point of water provides a convenient and reliable method for measuring pressure. By carefully controlling the temperature at this unique point, scientists can establish a precise pressure reference. This precision is crucial for calibrating pressure gauges and maintaining standards in various industries, such as manufacturing and meteorology.
In conclusion, the triple point of water is a captivating phenomenon that reveals the intricate nature of matter and its phase transitions. Its significance extends far beyond its theoretical implications, reaching into practical applications that shape our daily lives. As we continue to explore the complexities of the world around us, the triple point of water remains an intriguing and valuable reference point for scientists and engineers alike.
Calibrating Thermometers with the Triple Point of Water: A Tale of Precision and Accuracy
In the realm of temperature measurement, precision and accuracy are paramount. Thermometers play a crucial role in various scientific, industrial, and even daily tasks, and ensuring their reliability is vital. Enter the triple point of water, a remarkable phenomenon that serves as a fixed reference point for calibrating thermometers with unparalleled accuracy.
Defining the Triple Point
The triple point of a substance is the unique temperature and pressure at which its solid, liquid, and gaseous phases coexist in equilibrium. For water, this point occurs at 273.16 Kelvin (0.01°C) and a pressure of 611.657 pascals. At this precise point, all three phases exist simultaneously, creating a stable and consistent reference for temperature calibration.
The Role of the Triple Point in Calibration
Thermometers are calibrated by comparing their readings to a known reference temperature. The triple point of water provides such a reference with remarkable precision. By immersing a thermometer in a triple point cell, where water is maintained at its triple point, we can adjust the thermometer’s scale to align exactly with the standard temperature.
This calibration process ensures that the thermometer’s readings are accurate and reliable. Accurate temperature readings are crucial in many fields, including climate science, pharmaceutical research, and food safety. Inaccurate readings can lead to erroneous conclusions and potentially dangerous situations.
The Importance of Accuracy in Applications
Accurate temperature readings play a significant role in various applications:
- Medical Diagnostics: Precise temperature measurements are essential for diagnosing and monitoring conditions such as fever and hypothermia.
- Pharmaceutical Research: Accurate temperature control is crucial in manufacturing and testing pharmaceuticals, ensuring their stability and effectiveness.
- Climate Science: Temperature data is indispensable for studying climate change, weather forecasting, and understanding global climate patterns.
- Industrial Processes: Many industrial processes rely on precise temperature control for optimal efficiency and product quality.
By calibrating thermometers against the triple point of water, we can ensure the accuracy of these vital measurements and their impact on countless applications.
The Triple Point of Water and the Kelvin Scale: Defining the Absolute Zero
The triple point of water is a fascinating phenomenon where the solid, liquid, and gaseous phases of water coexist in perfect equilibrium at a remarkable temperature of 273.16 Kelvin (0.01 degrees Celsius) and a pressure of 611.73 pascals (0.006037306 atmospheres). This unique occurrence has significant implications for the scientific world.
The triple point of water plays a pivotal role in defining the Kelvin scale, the fundamental unit of temperature in the International System of Units (SI). By establishing a fixed reference point, it allows scientists to measure temperature with incredible precision. The Kelvin scale is an absolute temperature scale that begins at absolute zero, the theoretical point where all molecular motion ceases. This concept emerged from the study of thermodynamics, a branch of physics that explains the relationship between heat and other forms of energy. The Kelvin scale provides a universal standard for temperature measurement, eliminating discrepancies and enabling scientific comparisons across different fields.
Understanding the triple point of water and its relation to the Kelvin scale empowers us to appreciate the rigor and precision of scientific measurements. It represents a cornerstone in the field of thermometry, allowing scientists to accurately determine and compare temperatures, which is vital in numerous applications, ranging from medical diagnostics to industrial processes and climate science. By unraveling the mysteries of the triple point, we gain a deeper understanding of the fundamental properties of matter and the tools we use to explore our physical world.
Measuring Pressure using the Triple Point of Water
In the world of physics and engineering, precise pressure measurements are essential for various applications. One highly accurate and reliable method involves using the triple point of water.
What is the Triple Point?
The triple point is a unique combination of temperature and pressure at which a substance can coexist in three phases: solid, liquid, and gas. For water, the triple point occurs at 273.16 Kelvin (0.01°C) and 0.00603 kilopascals (kPa).
Triple Point as a Pressure Reference
The triple point of water provides a fixed reference point for pressure measurement. When water is brought to its triple point, it exists as ice, liquid water, and water vapor simultaneously. The pressure at this point is precisely defined and constant. By measuring the pressure at which water reaches its triple point, scientists and engineers can obtain an accurate and reproducible pressure reading.
Precision of Pressure Measurement
The triple point of water offers exceptional precision for pressure measurement. The pressure at the triple point is constant and remains unchanged regardless of external factors such as temperature fluctuations or variations in water purity. This makes it an ideal calibration standard for pressure gauges and transducers, ensuring accurate pressure readings in various applications.
Applications in Pressure Standards
The triple point of water has become the basis for pressure standards used worldwide. National and international metrology institutes maintain highly precise triple point cells to provide traceable pressure calibrations. These cells are used to calibrate a range of pressure measuring devices, from laboratory standards to industrial pressure gauges, ensuring the reliability and accuracy of pressure measurements across various fields.