Unveiling The Triple Bond: A Deep Dive Into Its Structure, Formation, And Significance
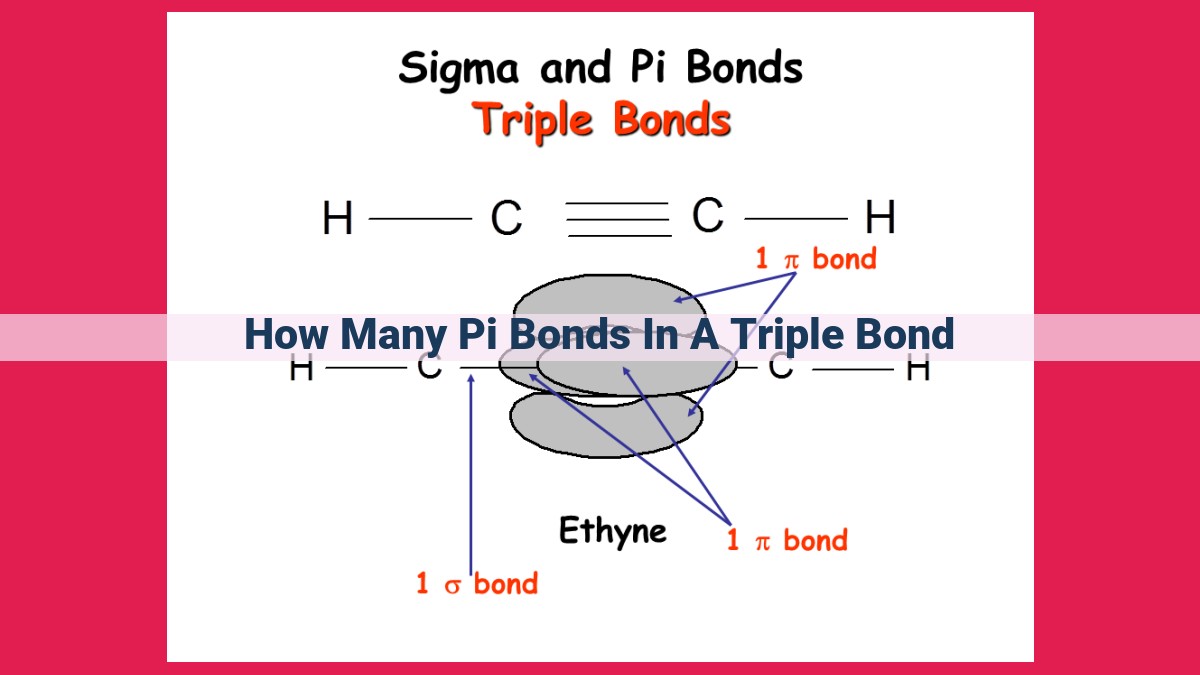
A triple bond, typically found in molecules like alkynes, consists of a combination of sigma and pi bonds. The sigma bond forms from the head-on overlap of atomic orbitals, while the pi bonds result from the lateral overlap of p-orbitals. Notably, a triple bond comprises two pi bonds, which contribute significantly to its overall strength and stability. The formation of triple bonds depends on factors such as electronegativity and the availability of valence electrons. Triple bonds play a crucial role in various molecules, impacting their polarity, reactivity, and molecular geometry.
Triple Bond: An Overview
In the captivating world of molecular chemistry, bonds between atoms play a crucial role in shaping the structure and properties of countless substances. Among these bonds, the triple bond stands out for its exceptional strength and significance. Delving into its captivating realm, we’ll unravel the essence of triple bonds, exploring their formation, types, and the profound impact they wield on molecules.
A triple bond is an extraordinary chemical bond that unites two atoms with three pairs of electrons, forging an exceptionally robust connection. This bond is not a simple entity but rather a dynamic interplay of different bond types, each contributing unique characteristics.
Sigma and Pi Bonds: A Cohesive Union
The triple bond is a harmonious union of a sigma bond and two pi bonds. The sigma bond, akin to an unwavering pillar, forms from the head-on overlap of atomic orbitals. The pi bonds, on the other hand, emerge from the lateral overlap of orbitals and flank the sigma bond, akin to steadfast guardians.
Two Pi Bonds: Unveiling the Strength
Intriguingly, triple bonds possess two pi bonds in their molecular architecture, adding an extra layer of strength to the overall bond. These pi bonds are perpendicular to the sigma bond, creating a stable, trigonal arrangement around the bonded atoms.
Factors Governing Formation: A Delicate Balance
The formation of triple bonds is governed by a delicate interplay of factors, including electronegativity and valence electrons. Atoms with high electronegativity, eager to attract electrons, are less inclined to form triple bonds. Conversely, atoms with numerous valence electrons, eager to share their electronic wealth, readily engage in triple bond formation.
Applications of Triple Bonds: A Versatile Force
Triple bonds are not mere theoretical curiosities; they play vital roles in countless molecules that underpin our world. From acetylene, the backbone of plastics, to carbon monoxide, a ubiquitous industrial gas, triple bonds are essential components of a vast array of compounds. Their unique properties make them indispensable in organic chemistry, materials science, and beyond.
Impact on Properties: Unveiling Molecular Secrets
Triple bonds exert a profound influence on molecular properties. They render molecules more polar due to the uneven distribution of electrons, enhance their reactivity by providing accessible electrons, and constrain molecular geometry by dictating specific bond angles. These effects shape the behavior and applications of molecules in countless fields.
Sigma and Pi Bonds: The Building Blocks of Triple Bonds
When it comes to chemical bonds, triple bonds are like the ultimate powerhouses, bringing molecules together with an unwavering grip. These extraordinary bonds are made up of two distinct components: sigma bonds and pi bonds, each playing a crucial role in the stability and properties of molecules.
Sigma Bonds: The Foundation of Every Bond
Imagine a sigma bond as a sturdy pillar holding two atoms together. This type of bond is formed by the head-on overlap of orbitals, creating a direct and strong connection between the atoms. Think of it as a straight line connecting two points, providing the backbone for many single and double bonds.
Pi Bonds: Adding Strength and Character
In contrast to sigma bonds, pi bonds are more like flexible dancers, swirling around the sigma bond like ribbons. They are formed by the lateral overlap of orbitals, creating a side-by-side connection between atoms. These bonds add extra stability and unique character to molecules, providing additional strength and influencing molecular properties.
In a triple bond, the unwavering strength of a sigma bond is complemented by the agile support of two pi bonds. This trio of bonds creates an incredibly strong connection, making triple bonds one of the most powerful bonding arrangements in chemistry. They are the backbone of molecules like acetylene and carbon dioxide, contributing to their stability and reactivity.
Understanding the nature of sigma and pi bonds is essential for comprehending the behavior of molecules. These bonds, particularly the double pi bonds in triple bonds, dictate molecular polarity, reactivity, and molecular geometry. They are the foundation upon which the vast world of chemistry is built, shaping the properties and interactions of countless molecules that make up our world.
The Number of Pi Bonds in a Triple Bond: Delving into Molecular Architecture
Every chemist worth their salt knows that the formation of chemical bonds is the backbone of molecular existence. Among these bonds, the triple bond stands out as a formidable force, holding atoms together with an unyielding grip. But what exactly is a triple bond, and how does it differ from its single and double counterparts?
Understanding the Essence of a Triple Bond
At its core, a triple bond is an extraordinary union between two atoms, held together by a formidable force field of three electron pairs. This extraordinary bond is a testament to chemistry’s relentless pursuit of stability, as it offers a level of bonding strength unmatched by its single and double bond brethren.
Sigma and Pi: The Symbiotic Bond Duo
Within the triple bond’s embrace lies a harmonious coexistence of two bond types: the sigma bond and the enigmatic pi bond. The sigma bond, a pillar of strength, is formed by the head-on overlap of atomic orbitals, creating a direct line of communication between the bonded atoms. This unwavering bond forms the foundation of the triple bond’s resilience.
Complementing the sigma bond’s unwavering presence are two pi bonds, adding an extra layer of bonding prowess to the triple bond’s arsenal. These pi bonds arise from the lateral overlap of atomic orbitals, creating a sideways embrace that further solidifies the bond between the atoms.
The Significance of Two Pi Bonds
The presence of two pi bonds is not merely an accessory; it’s the very essence of a triple bond’s exceptional strength. These pi bonds entwine the atoms in a dance of shared electrons, amplifying the bond’s stability and imbuing the molecule with unique properties.
The triple bond, with its remarkable assembly of three electron pairs, emerges as a pivotal player in the realm of molecular chemistry. Its unique composition of two pi bonds, interwoven with a resolute sigma bond, elevates it to the pinnacle of bond strength. This formidable bond finds its place in a myriad of molecules, showcasing its versatility and importance in shaping the world around us.
Formation of Triple Bonds: The Key to Unlocking Molecular Complexity
In the realm of molecular chemistry, triple bonds emerge as intricate and fascinating entities, playing a pivotal role in shaping the properties and behavior of molecules. Understanding their formation unravels the secrets of chemical bonding and molecular architecture.
Electronegativity’s Dance: A Bond-Influencing Factor
The dance of electronegativity, a measure of an atom’s ability to attract electrons, significantly influences the formation of triple bonds. When atoms with similar electronegativity encounter each other, their shared electrons reside in a relatively equal dance, allowing for the formation of strong, stable triple bonds.
Valence Electrons: The Building Blocks of Bonding
Valence electrons, the outermost electrons of an atom, play a crucial role in determining whether a triple bond can form. For instance, carbon atoms, with four valence electrons, can readily form triple bonds with themselves or other atoms, such as nitrogen, which also possesses three valence electrons. This electronic harmony leads to the creation of molecules like acetylene (C2H2), the simplest alkyne molecule with a carbon-carbon triple bond.
Overcoming the Energy Barrier: Hybrid Orbitals to the Rescue
The formation of triple bonds requires overcoming a significant energy barrier. Here, the concept of hybrid orbitals comes into play. Hybrid orbitals, a combination of atomic orbitals, allow for a more efficient overlap of electron clouds, facilitating the formation of stronger bonds. In the case of triple bonds, sp-hybridized orbitals form the sigma bond, while two sets of p-orbitals overlap sideways to create two pi bonds, resulting in the characteristic triple bond.
By delving into the formation of triple bonds, we unlock a deeper understanding of molecular complexity. From electronegativity’s dance to valence electrons’ harmony and the magic of hybrid orbitals, the intricate processes that govern triple bond formation lay the foundation for the diverse molecular world we inhabit.
Applications of Triple Bonds: Unlocking Versatile Molecular Structures and Properties
Triple bonds, characterized by their unrivaled strength and distinct molecular properties, find widespread applications across various scientific disciplines, particularly in organic chemistry and materials science. These versatile bonds contribute to the formation of molecules with exceptional stability, reactivity, and functionality.
One prominent example of a molecule containing triple bonds is acetylene (C2H2), the simplest alkyne hydrocarbon. Acetylene’s triple bond between the two carbon atoms imparts high reactivity, enabling its use as a starting material for the synthesis of a vast array of organic compounds, including plastics, pharmaceuticals, and dyes.
In the realm of materials science, triple bonds play a crucial role in the development of high-strength materials, such as carbon nanotubes and graphene. These materials, composed of carbon atoms arranged in a hexagonal lattice with triple bonds, exhibit exceptional mechanical properties, making them ideal for applications in aerospace, electronics, and biomedical devices.
Additionally, triple bonds contribute to the conductivity of certain organic molecules, such as polyynes. These molecules, featuring alternating single and triple bonds between carbon atoms, display semi-conducting properties, making them promising candidates for use in electronic devices.
Furthermore, the unique molecular geometry associated with triple bonds influences the reactivity and selectivity of chemical reactions. For instance, triple bonds in unsaturated fatty acids undergo a variety of reactions, including hydrogenation and epoxidation, which are essential for the production of essential oils and other value-added products.
In conclusion, triple bonds serve as fundamental building blocks in the construction of molecules with diverse properties and applications. Their unique strength, reactivity, and molecular geometry have made them indispensable tools in fields ranging from organic chemistry to materials science.
Properties of Molecules with Triple Bonds
Triple bonds, composed of a sigma bond and two pi bonds, significantly influence the properties of molecules. These bonds impart unique characteristics that distinguish molecules with triple bonds from those with other types of bonds.
Molecular Polarity:
Triple bonds, due to their electron-rich nature, contribute to the polarity of molecules. The strong electronegative forces in the pi bonds draw electrons toward them, creating a polarization within the molecule. This polarity affects the molecule’s interactions with other polar molecules, influencing its solubility, reactivity, and behavior in different environments.
Reactivity:
Molecules with triple bonds are generally more reactive than those with single or double bonds. The two energetically accessible pi bonds provide opportunities for electrons to be shared or rearranged, facilitating chemical reactions. This reactivity makes molecules with triple bonds useful in various chemical processes, including those used in the synthesis of new compounds.
Molecular Geometry:
Triple bonds have a profound impact on molecular geometry. The linear shape of the triple bond forces the adjacent atoms to be oriented in a straight line. This linear geometry affects the molecule’s overall shape and influences its physical and chemical properties.
In summary, understanding the impact of triple bonds on molecular properties is crucial for comprehending the behavior of molecules and their applications in various areas of chemistry. The unique characteristics imparted by triple bonds, including their electron-rich nature, polarity, reactivity, and molecular geometry, make them essential components in understanding the intricate world of chemical bonding and molecular structure.