Understanding D-Electron Count: Key To Unlocking The Behavior Of Transition Metals
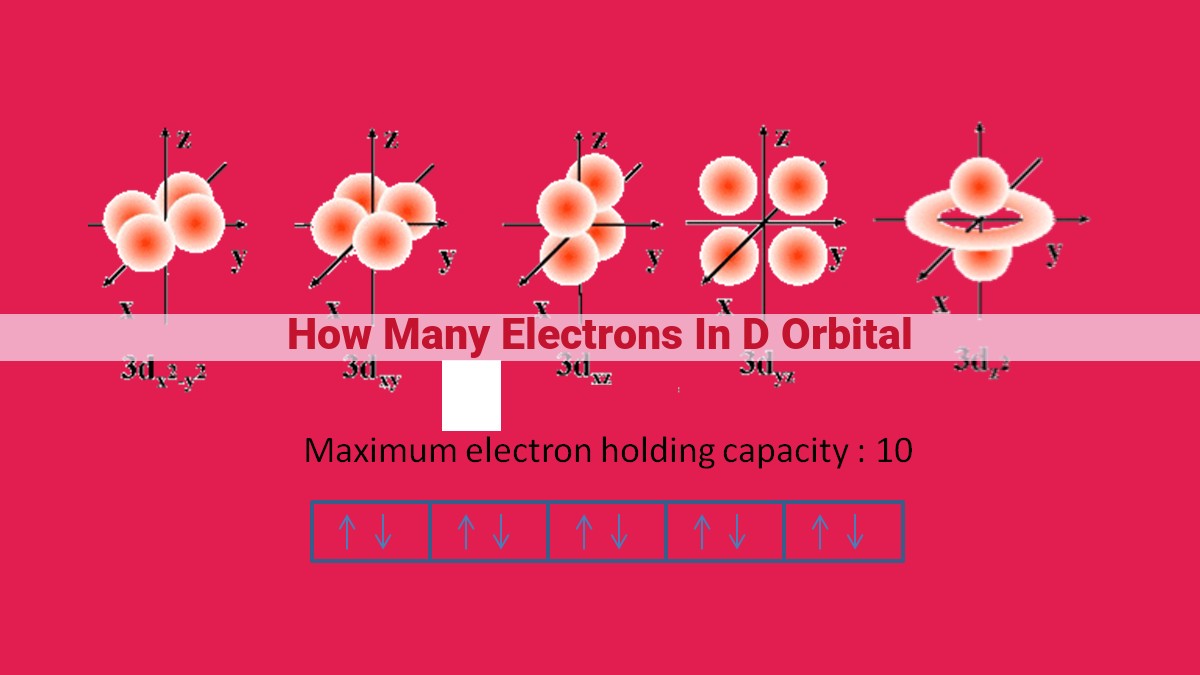
According to the Aufbau principle, d orbitals have a capacity of 10 electrons. The number of d electrons varies from 1 to 10 in transition metals, with each orbital holding a maximum of two electrons (Hund’s rule). The d electron count significantly influences their properties, including color, magnetism, catalytic activity, and ability to form complexes. Understanding the number of d electrons is crucial for predicting and explaining the behavior of transition metals in various chemical and industrial applications.
Unveiling the Secrets of Electron Arrangement: The Aufbau Principle
In the realm of atoms, the Aufbau principle reigns supreme, guiding the enigmatic dance of electrons. It dictates the orderly arrangement of these subatomic particles within atomic orbitals, the energy levels that govern their behavior.
The Aufbau principle dictates that electrons fill orbitals in a hierarchical fashion, starting with the lowest energy orbitals and working their way up. The energy of an orbital is determined by its shape and proximity to the atomic nucleus. Filling an orbital of lower energy requires less energy, making it more favorable for electrons.
The starting point for electron occupation is the 1s orbital, the orbital closest to the nucleus and with the lowest energy. From there, electrons progressively fill the 2s, 2p, 3s, 3p, and so on, in ascending order of energy. This ordered occupancy of orbitals ensures the most stable electronic configuration for an atom.
By understanding the Aufbau principle, we gain a deeper insight into the electronic structure of atoms and the foundation for comprehending their properties and behavior.
Hund’s Rule: Unraveling the Secrets of Electron Spin Configuration
In the captivating realm of atomic chemistry, electrons dance gracefully around the nucleus, occupying specific energy levels known as orbitals. One of the key principles guiding their arrangement is Hund’s rule, named after the illustrious physicist Friedrich Hund. This rule dictates the preferred spin configuration of electrons in degenerate orbitals, shaping the behavior of atoms and molecules.
What is Hund’s Rule?
Hund’s rule states that when multiple electrons occupy degenerate orbitals (having the same energy), they will maximize their spin. This means that they will prefer to have parallel spins before pairing up with opposite spins. Imagine two electrons entering degenerate orbitals like two dancers on a crowded dance floor. Instead of immediately pairing up, they initially choose to dance independently with the same spin direction.
Significance of Hund’s Rule
Understanding Hund’s rule is crucial because it determines the overall spin multiplicity of atoms and molecules. Spin multiplicity refers to the number of possible spin states for a given configuration. For example, an atom with two unpaired electrons has a spin multiplicity of 3 (triplet state), while an atom with two paired electrons has a spin multiplicity of 1 (singlet state).
Examples of Hund’s Rule in Action
Consider the following examples to solidify our understanding:
- Nitrogen atom (N): With atomic number 7, nitrogen has five electrons, two of which occupy the degenerate 2p orbitals. According to Hund’s rule, these two electrons will have parallel spins, leading to a triplet state.
- Oxygen atom (O): With eight electrons, oxygen has two electrons in each of the 2p orbitals. Again, Hund’s rule dictates that these electrons will have parallel spins, resulting in a triplet state for oxygen as well.
Hund’s rule plays a vital role in shaping electron configurations and determining the spin properties of atoms and molecules. By maximizing the spin of electrons in degenerate orbitals, it influences the chemical reactivity and magnetic behavior of substances. Understanding this rule is fundamental to comprehending the intricate dance of electrons and the mysteries of atomic physics.
d Orbital: Shape and Characteristics:
- Define a d orbital and its unique shape.
- Describe the geometric properties and energy levels associated with d orbitals.
- Explain the sublevel designation (l = 2) and its implications for electron occupancy.
Exploring the Enigmatic World of d Orbitals: Unraveling Their Shape and Characteristics
As we delve into the realm of atomic structure, we encounter the fascinating world of orbitals, where electrons reside like celestial bodies. Among these orbitals, one intriguing species stands out: d orbitals. This blog post embarks on an adventure to uncover their unique shape and characteristics, providing an in-depth understanding of these fundamental building blocks of matter.
What is a d Orbital?
Picture a d orbital as a four-lobed, three-dimensional structure with two ring-shaped lobes lying perpendicular to each other. These lobes resemble miniature doughnuts, each with a hole in the center. This peculiar shape arises from the intricate interactions between electrons and the nucleus.
Geometric Properties and Energy Levels
The geometric arrangement of d orbitals is governed by their sublevel designation (l = 2). This indicates that they reside in the third energy level of an atom. Each energy level is further divided into sublevels with increasing energy. For d orbitals, the five sublevels are denoted as dxy, dyz, dxz, dx^2-y^2, and dz^2.
The Aufbau principle dictates the order in which these sublevels are filled with electrons. Starting from the lowest energy sublevel (dxy and dyz), electrons are sequentially added to each until they reach the highest energy sublevel (dz^2).
Implications for Electron Occupancy
The unique shape of d orbitals has profound implications for electron occupancy. According to Hund’s rule, electrons prefer to occupy separate orbitals within the same sublevel with parallel spins before pairing. This results in a maximum number of unpaired electrons, which contributes to the magnetic properties of atoms and molecules.
d Orbitals play a pivotal role in the chemistry of transition metals. Their distinct shape and energy levels influence reactivity, coordination behavior, and magnetic properties. Understanding the characteristics of d orbitals is crucial for comprehending the complex world of atomic structure and its impact on the chemical world. By unraveling the mysteries of these enigmatic orbitals, we gain a deeper appreciation for the fundamental principles that govern the nature of matter.
d Orbital Electron Counts and Transition Metal Chemistry
The world of chemistry revolves around the intricate dance of electrons within atoms. Transition metals, with their captivating d orbitals, take this dance to an entirely new level. These orbitals, with their unique shapes and energy profiles, play a pivotal role in shaping the reactivity and behavior of these remarkable elements.
The number of d electrons in a transition metal is a key factor that influences its properties. As we move across the periodic table from left to right, the number of d electrons increases from 1 to 10. This gradual increase has profound implications for the chemistry of these elements.
Transition metals with a low number of d electrons (1-4) tend to be more reactive and form ionic compounds. They exhibit a strong tendency to lose electrons to achieve a stable electronic configuration. For instance, iron(II) (Fe²⁺) readily forms ionic bonds with negative ions like chloride (Cl⁻) to form compounds like FeCl₂.
In contrast, transition metals with a high number of d electrons (6-10) are typically less reactive and form covalent compounds. They prefer to share electrons with other atoms to form stable bonds. For example, chromium(VI) (Cr⁶⁺) readily forms covalent bonds with oxygen atoms to form compounds like CrO₄²⁻.
The number of d electrons also governs the magnetic properties of transition metals. Metals with an odd number of d electrons are paramagnetic, meaning they are attracted to magnetic fields. This is because the unpaired electrons in the d orbitals can align with an external magnetic field. On the other hand, metals with an even number of d electrons are diamagnetic, meaning they are not attracted to magnetic fields.
Furthermore, the number of d electrons influences the catalytic activity of transition metals. Many transition metals are excellent catalysts, facilitating chemical reactions without being consumed themselves. The d electrons provide a versatile platform for bonding with reactants and intermediates, enabling efficient catalytic processes.
In summary, the number of d electrons in a transition metal is a critical determinant of its reactivity, behavior, and applications. Understanding this concept is essential for comprehending the fascinating world of transition metal chemistry and its technological significance.
d Electrons: Shaping the Properties of Transition Metals
In the realm of chemistry, the nature and number of electrons that reside in an atom’s outermost orbitals, known as d electrons, play a pivotal role in determining the unique properties and versatile applications of transition metals. These remarkable elements form the heart of modern technology, from smartphones to advanced energy systems.
Physical and Chemical Properties
The number of d electrons directly influences the physical and chemical properties of transition metals. Metals with fewer d electrons (d⁴ or less) generally exhibit higher melting and boiling points due to stronger metallic bonding, making them more resistant to heat and deformation. In contrast, metals with more d electrons (d⁵ or above) tend to have lower melting and boiling points, rendering them softer and more easily malleable.
Coordination Chemistry
d Electrons are the key players in coordination chemistry, the study of metal complexes formed when transition metal ions bind to ligands (molecules or ions) through coordinate covalent bonds. The number and arrangement of d electrons determine the geometry and stability of these complexes, which are essential for many biochemical processes and industrial applications.
Catalysis
Transition metals are widely employed as catalysts, substances that accelerate chemical reactions without being consumed themselves. The d electrons enable them to interact with reactants, providing activation energy pathways that reduce the time and energy required for reactions to occur. This catalytic prowess finds applications in a myriad of industries, including refining, pharmaceuticals, and environmental protection.
Material Science
d Electrons also contribute to the electronic band structure of transition metals, influencing their electrical conductivity, magnetism, and optical properties. These characteristics make them invaluable for electronics, magnets, and superconductors, among other advanced materials. For example, the unique magnetic properties of transition metals are exploited in hard disk drives and magnetic resonance imaging (MRI) devices.
Applications in the Real World
- d⁴ Configuration: Titanium with its four d electrons is a lightweight metal used in aerospace and medical implants due to its high strength-to-weight ratio and biocompatibility.
- d⁶ Configuration: Cobalt with its six d electrons is a vital component in lithium-ion batteries, where it provides electrochemical stability and long cycle life.
- d¹⁰ Configuration: Copper with its fully occupied d orbitals is an excellent electrical conductor used in electrical wiring, electronics, and renewable energy systems.
In conclusion, d electrons are the driving force behind the unique properties and versatile applications of transition metals. Their number and arrangement govern everything from physical characteristics and catalytic abilities to electronic behavior and material properties. Understanding the role of d electrons is crucial for unlocking the full potential of these remarkable elements in shaping the world around us.