Unlocking Titanium’s Valence Electron Mystery: Unraveling Its Chemical Reactivity
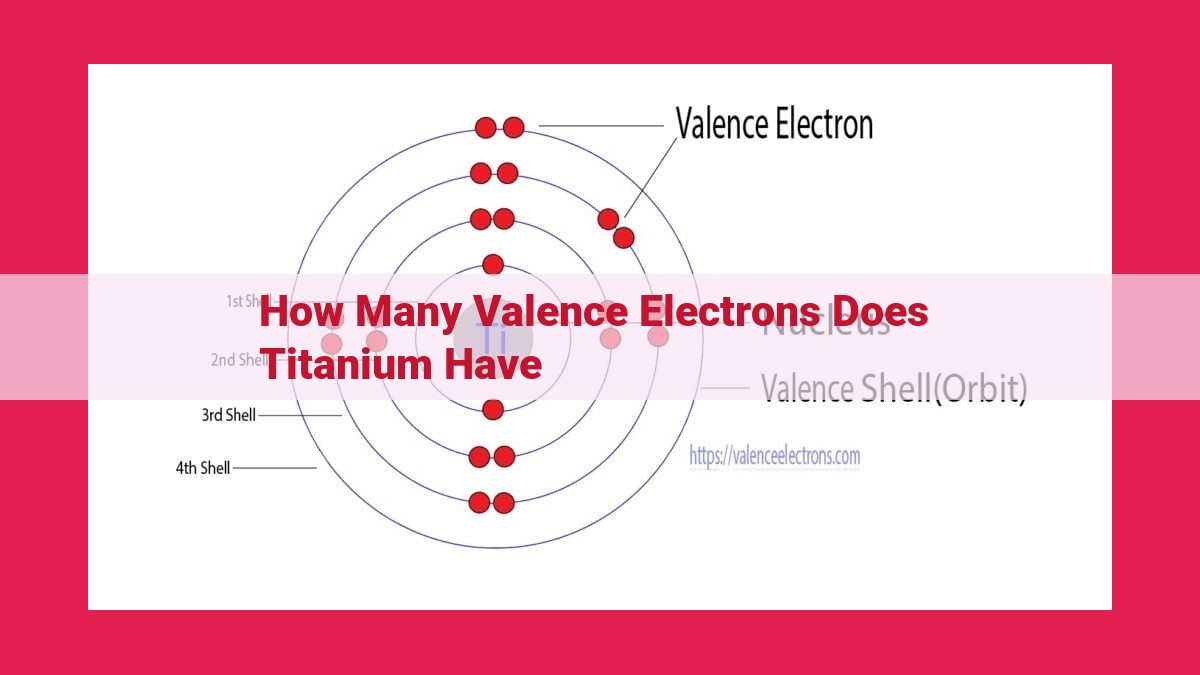
Titanium, a transition metal in Group 4, has a unique electron configuration that determines its valence electrons. By building the electron configuration of titanium using atomic orbitals and the Aufbau principle, we can calculate the number of valence electrons. Titanium has 4 valence electrons, which are located in its outermost shell and play a significant role in its chemical bonding behavior. These valence electrons allow titanium to form various bonds with other atoms, influencing its properties and reactivity.
- Define valence electrons and their significance in chemical reactions.
- Explain the octet rule and its role in valence electron configuration.
- Discuss Lewis structures as a representation of valence electrons in covalent bonds.
In the fascinating world of chemistry, valence electrons hold the key to understanding how elements interact and form bonds. These are the electrons that reside in an atom’s outermost energy level, the ones that dance around the nucleus, eager to make their mark. They play a pivotal role in chemical reactions, shaping the properties and behavior of substances.
One fundamental concept in chemistry is the octet rule, which states that atoms tend to gain or lose electrons to achieve a stable configuration with eight valence electrons. It’s like a cosmic dance, where atoms strive to complete their “octet” of valence electrons by forming covalent bonds. In covalent bonds, atoms share electrons, a beautiful collaboration that holds molecules together.
Chemists use a visual tool called Lewis structures to depict the arrangement of valence electrons in covalent bonds. These structures are like chemical maps, revealing the connections between atoms and providing insights into their electronic behavior. By studying valence electrons and their interactions, we unravel the secrets of chemical bonding and gain a deeper understanding of the molecular world around us.
Titanium: A Versatile Transition Metal
In the realm of elements, titanium stands out as a remarkable metal with unique properties that make it indispensable in various industries. As a transition metal, it occupies a pivotal position in Group 4 of the periodic table. This strategic placement profoundly influences its chemical behavior and grants it an array of versatile applications.
Titanium derives its name from the Titans, the mythological giants of Greek folklore. Its symbol, Ti, symbolizes its exceptional strength and durability. With an atomic number of 22, titanium boasts 22 protons and 22 electrons, maintaining a neutral charge. Its atomic mass of approximately 47.9 amu reflects the presence of 26 neutrons within its nucleus.
The position of titanium in Group 4 signifies that it possesses four valence electrons. Valence electrons play a crucial role in chemical bonding, determining an element’s reactivity and the types of compounds it can form. In titanium’s case, these four valence electrons enable it to participate in a wide range of chemical reactions, forming strong bonds with various elements.
Titanium’s chemical behavior is further influenced by its atomic mass. The relatively high atomic mass of titanium indicates a large nucleus, which exerts a strong attractive force on the surrounding electrons. This strong nuclear pull results in a stable electron configuration, making titanium less reactive than some other transition metals.
In summary, titanium’s position as a transition metal in Group 4, its atomic number of 22, and its atomic mass of 47.9 amu collectively shape its chemical properties. These characteristics endow titanium with a unique combination of strength, durability, and versatility, making it an essential material in industries ranging from aerospace to biomedical engineering.
Determining Valence Electrons
- Explain the concept of atomic orbitals, quantum numbers, and the Aufbau principle.
- Provide a step-by-step process for building the electron configuration of titanium.
- Calculate the number of valence electrons based on the electron configuration.
Determining Valence Electrons: A Step-by-Step Guide
Introduction
Imagine yourself as a chemical detective, tasked with uncovering the secrets of the element titanium. To understand how it behaves in the chemical world, we must first unravel the mystery of its valence electrons.
Atomic Orbitals and Quantum Numbers
Picture atoms as tiny solar systems. The electrons in these systems occupy specific atomic orbitals, defined by three quantum numbers:
- Principal Quantum Number (n): Describes the shell in which the electron is found.
- Azimuthal Quantum Number (l): Represents the shape of the orbital (e.g., s, p, d, f).
- Magnetic Quantum Number (ml): Specifies the orientation of the orbital in space.
Aufbau Principle
The Aufbau principle guides us in filling these orbitals. Electrons fill the lowest-energy orbitals first, following a specific order:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p
Building Titanium’s Electron Configuration
Let’s embark on a journey to construct the electron configuration of titanium, which has an atomic number of 22. This number tells us it has 22 electrons to fill its orbitals.
- First Shell: 2 electrons occupy the 1s orbital (n=1, l=0).
- Second Shell: 8 electrons occupy the 2s (n=2, l=0) and 2p (n=2, l=1) orbitals.
- Third Shell: 10 electrons occupy the 3s (n=3, l=0), 3p (n=3, l=1), and 3d (n=3, l=2) orbitals.
Valence Electrons
The outermost electrons, those in the highest occupied shell, are known as valence electrons. They determine an element’s chemical reactivity and bonding behavior. By counting the electrons in the 3s and 3d orbitals, we find that titanium has 4 valence electrons.
Atomic Number and Element Symbol: Unraveling the Identity of Titanium
The atomic number of an element is a fundamental property that defines its very essence. It represents the number of protons residing within the atomic nucleus. Each proton carries a positive charge, establishing the _element’s identity_. The _element symbol_ is a one- or two-letter shorthand notation that represents the element, with its _first letter always capitalized_.
When it comes to titanium, its atomic number is 22_. This means that every titanium atom has _22 protons in its nucleus. The element symbol for titanium is _Ti_. It is a simple yet powerful representation, capturing the unique characteristics of this versatile metal.
However, the story doesn’t end there. Titanium also exhibits isotopes_, which are atoms of the same element with the _same atomic number but _different numbers of neutrons_. Neutrons are neutral particles found in the nucleus alongside protons. Titanium has several isotopes, including _titanium-46, titanium-47, and titanium-48_. These isotopes differ in their neutron count, affecting their mass but not their chemical properties.
So, by delving into the atomic number and element symbol of titanium, we gain valuable insights into its identity and composition. These building blocks form the foundation for understanding titanium’s unique properties and its behavior in the fascinating world of chemistry.
Number of Valence Electrons in Titanium
Unveiling the Chemical Secrets of Titanium
In our exploration of valence electrons, we’ve delved into the fascinating world of chemical bonding. Now, let’s turn our attention to a specific element: titanium. This enigmatic metal holds a key to unlocking the secrets of chemical behavior.
Determining Titanium’s Electron Configuration
To understand titanium’s valence electrons, we first need to unravel its electron configuration. This roadmap of electrons reveals the number and arrangement of electrons within the element’s atomic orbitals. Following the Aufbau principle, we systematically fill these orbitals until we reach titanium’s atomic number, which is 22.
1s² 2s² 2p⁶ 3s² 3p⁶ 3d² 4s²
Counting Valence Electrons
The valence electrons are the electrons in the outermost shell of an atom. In titanium’s case, these are the two electrons in the 4s orbital. These valence electrons play a crucial role in determining the element’s chemical reactivity.
Implications for Chemical Bonding
The number of valence electrons in titanium has a profound impact on its bonding behavior. With two valence electrons, titanium typically forms covalent bonds, sharing electrons with other atoms to achieve a stable electron configuration. This explains why titanium is often found in compounds such as TiO₂, where it shares its valence electrons with oxygen atoms.
Valence electrons are the architects of chemical bonding, shaping the properties and reactivity of elements. In the case of titanium, its two valence electrons dictate its preference for covalent bonding, leading to the formation of compounds like TiO₂. Understanding valence electrons is essential for comprehending the intricate world of chemical interactions.