Determining Threshold Frequency Through Photoelectric Effect Experiment: A Comprehensive Guide
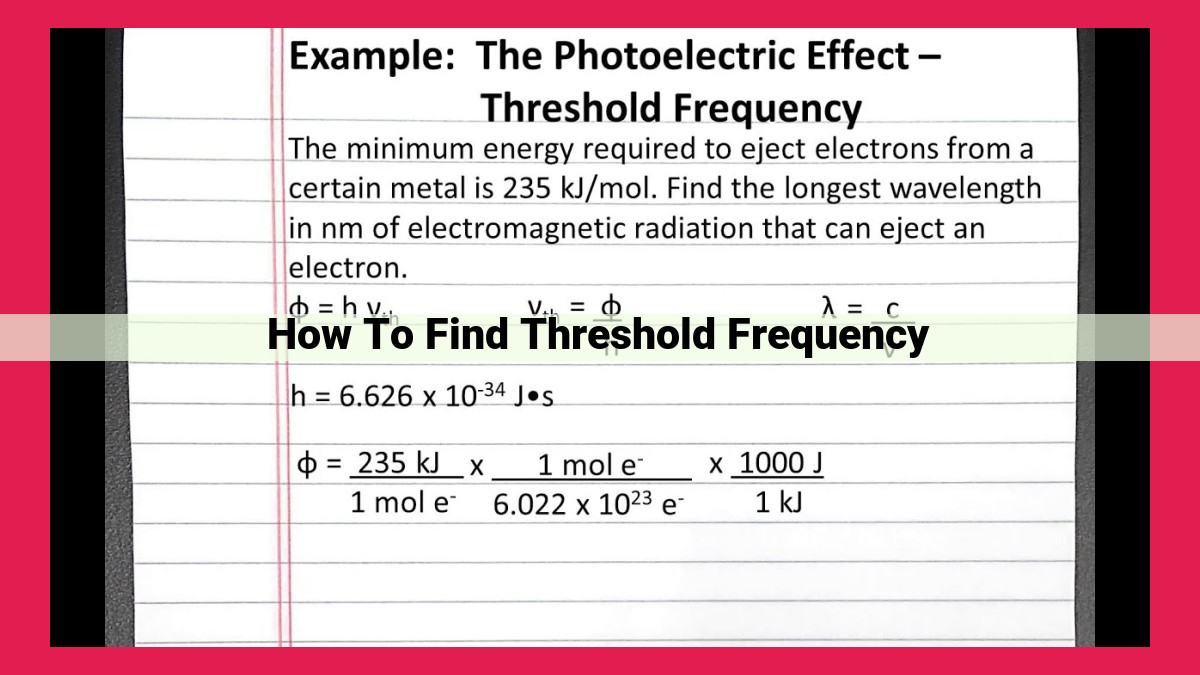
To find the threshold frequency of a material, you need to conduct the photoelectric effect experiment. Illuminate the material with incident light of varying frequencies. Record the maximum kinetic energy of the emitted electrons for each frequency. Plot a graph of maximum kinetic energy versus frequency. The threshold frequency is the frequency at which the maximum kinetic energy becomes zero. This threshold frequency corresponds to the minimum energy required to eject electrons from the material and is determined by the material’s work function. Einstein’s equation for threshold frequency, hf = Φ, provides a precise relationship between the threshold frequency, h (Planck’s constant), and Φ (the work function).
- Define incident light and its characteristics (wavelength, frequency, intensity)
- Explain the wave-particle duality of light
Incident Light: Understanding the Essence of Light’s Journey
In the realm of light, incident light holds a pivotal role. It is the electromagnetic radiation that strikes a surface, initiating a cascade of physical phenomena that shape our perception and interaction with the world around us.
Imagine a beam of sunlight streaming through a window, its wavelengths and frequencies dancing in harmony, the intensity of its rays illuminating the room. This is incident light, carrying with it a wave-particle duality.
As a wave, incident light exhibits properties such as diffraction and interference, creating colorful patterns when passing through narrow apertures. Yet, it also possesses particle-like behavior, manifesting as photons, discrete packets of energy. This dual nature is a defining characteristic of light, a fundamental duality that continues to fascinate scientists and inspire technological advancements.
Reflection of Incident Light: A Guiding Light through Surfaces
Light, a captivating phenomenon that paints our world with vibrant colors, embarks on a fascinating journey as it interacts with surfaces. One of its fundamental adventures is the act of reflection, where light bounces back from an encounter with a material. This mirror-like behavior not only allows us to gaze at our reflections but also reveals the nature of the surface itself.
The law of reflection governs this interaction with unwavering precision. It states that the angle of incidence (the angle at which light strikes the surface) is equal to the angle of reflection (the angle at which light bounces back). This principle ensures that the path of light remains symmetrical, as if reflected by an invisible mirror.
Surfaces, however, can exhibit diverse personalities when it comes to reflection. Some, like a polished mirror, display specular reflection, resulting in a sharp, well-defined reflection. This type of reflection gives us clear images and allows us to admire our sharp reflections.
On the other hand, surfaces with uneven textures or microscopic irregularities showcase diffuse reflection. Light scattering in multiple directions creates a soft, scattered glow, like that of a matte painting or a chalky surface. It disperses light, illuminating a wider area with a more subdued effect.
Understanding the nature of reflection has profound implications in our daily lives. From the shiny gleam of polished surfaces to the soft glow of matte paints, reflection shapes our perception of the world. It also plays a crucial role in technologies such as mirrors, telescopes, and optical instruments, enabling us to control and manipulate light for various applications.
Refraction of Incident Light: Unraveling the Bending of Light
In the realm of optics, the phenomenon of refraction takes center stage, captivating us with its ability to bend light as it transitions between different materials. This mind-bending effect has profound implications, influencing a myriad of everyday occurrences and technological advancements.
The index of refraction, a dimensionless quantity, quantifies the extent to which light bends. It represents the ratio of the speed of light in a vacuum to the speed of light in the medium. A higher index of refraction indicates a greater ability to bend light.
At the heart of refraction lies the concept of Snell’s Law, a fundamental principle that governs the behavior of light as it crosses the boundary between two distinct media. According to Snell’s Law, the angle of incidence, defined as the angle between the incoming light ray and the normal (perpendicular line) to the interface, is related to the angle of refraction, or the angle between the refracted light ray and the normal. The relationship is expressed as:
n1 * sin(θ1) = n2 * sin(θ2)
where n1 and n2 represent the indices of refraction of the first and second media, respectively, and θ1 and θ2 denote the angles of incidence and refraction.
This equation captures the essential interplay between the indices of refraction and the angles of incidence and refraction. If light travels from a medium with a higher index of refraction to one with a lower index, it will refract away from the normal. Conversely, if light travels from a medium with a lower index of refraction to one with a higher index, it will refract towards the normal.
Refraction manifests in a myriad of commonplace phenomena. For instance, the apparent bending of a straw submerged in a glass of water is caused by the different speeds of light in air and water. Similarly, the formation of a rainbow results from the refraction of sunlight as it traverses through raindrops.
In the realm of optics, refraction plays a pivotal role in the functioning of lenses, prisms, and a vast array of optical devices. Lenses, by manipulating the refraction of light, can focus or diverge light rays, enabling us to see objects at different distances. Prisms, on the other hand, can decompose white light into its constituent colors, revealing the rainbow spectrum.
Unveiling the mysteries of refraction not only expands our understanding of the physical world but also paves the way for advancements in optics and imaging technologies. From everyday gadgets to cutting-edge scientific instruments, the manipulation of light through refraction continues to shape our world in countless ways.
Diffraction of Incident Light
- Briefly explain the concept of diffraction and its effects
- Describe how diffraction affects the appearance of light passing through narrow apertures
Diffraction: The Dance of Light through Narrow Passages
Imagine a gentle beam of light, like a delicate ballerina, approaching a narrow opening. As it gracefully glides through the aperture, something magical happens. The light’s path is transformed, bending and spreading as if it were dancing through a shimmering veil. This enchanting phenomenon is known as diffraction.
Diffraction is the bending of light as it passes through a small opening or around an obstacle. The wavelength of the light plays a crucial role, with shorter wavelengths exhibiting more pronounced diffraction effects. As light waves encounter the edges of the opening, they spread out, creating a pattern of bright and dark bands beyond the aperture.
These bands are the result of the constructive and destructive interference between different parts of the light wave. When crests (peaks) of waves line up, they reinforce each other, creating a bright band. Conversely, when crests and troughs (valleys) overlap, they cancel each other out, giving rise to a dark band.
The diffraction pattern that emerges depends on the size and shape of the aperture. Narrower apertures lead to wider diffraction patterns, while wider apertures produce narrower patterns. This effect can be seen in everyday life, such as when sunlight shines through gaps between blinds or creates colorful rainbows around raindrops.
Diffraction has numerous applications in science and technology. It is used in telescopes to enhance image resolution, in lasers to create highly focused beams, and in optical microscopy to study microscopic structures. By understanding the principles of diffraction, scientists and engineers can harness the power of light to reveal hidden details and push the boundaries of knowledge.
Transmission of Light
- Define transmission and discuss factors affecting it
- Explain how refraction affects the speed of light in different media
Transmission of Light: The Journey of Photons through Matter
Light, a fascinating manifestation of energy, interacts with matter in various ways. Transmission occurs when light passes through a medium, encountering atoms and molecules but continuing its journey. This remarkable phenomenon is influenced by the medium’s properties, making it a captivating subject for scientific exploration.
Factors Affecting Transmission
The transmission of light depends on several key factors:
- Material’s Density: Denser materials have a greater number of atoms and molecules per unit volume, increasing the likelihood of light scattering or absorption.
- Wavelength of Light: The wavelength of light, which determines its energy and properties, also affects transmission. Shorter wavelengths are more susceptible to scattering and absorption compared to longer wavelengths.
- Surface Roughness: Rough surfaces cause light to scatter in multiple directions, reducing transmission. Smoother surfaces, on the other hand, facilitate more direct transmission with minimal scattering.
Refraction and the Speed of Light
As light travels from one medium to another, it encounters a change in speed due to the medium’s refractive index. This index is a measure of how much the medium slows down light compared to the speed of light in a vacuum. When light enters a denser medium, its speed decreases, causing it to bend or refract. This phenomenon is commonly observed when a light beam passes from air into water.
Unveiling the Enigmatic World of Absorption: Where Light Vanishes
In the realm of light’s interactions with matter, absorption emerges as a captivating phenomenon where photons, the fundamental units of light, meet their fate. Unlike reflection or refraction, absorption is the process by which light is captured, its energy converted into a different form.
How Does Absorption Work?
When light impinges upon a material, its photons encounter electrons orbiting the atoms. If the energy of a photon matches the excitation energy of an electron, the photon is absorbed, and the electron transitions to a higher energy level. This energy transfer is like a perfect dance, where the photon’s energy is akin to the precise steps needed to lift the electron to a new quantum realm.
Effects of Absorption
The absorption of light can have profound effects on the material’s properties. It can cause:
- Heating: The absorbed energy can be dissipated as heat, raising the temperature of the material.
- Chemical Changes: Absorption can trigger chemical reactions, as the absorbed energy breaks or forms chemical bonds.
- Biological Processes: In living organisms, absorption plays crucial roles in processes like photosynthesis and vision.
Applications of Absorption
The science of absorption has countless practical applications:
- Solar Energy: Solar panels rely on the absorption of sunlight to generate electricity.
- Filters: Colored filters selectively absorb specific wavelengths of light, creating diverse hues and patterns.
- Medical Imaging: X-ray and MRI scanners utilize absorption to generate images of the body’s internal structures.
- Optical Communications: Fiber optic cables use absorption to prevent light from escaping, enabling high-speed data transmission.
Absorption is an essential phenomenon that unveils the intricate interplay between light and matter. It transforms light energy into other forms, shaping the properties of materials and driving a myriad of technological and biological processes. By delving into the depths of absorption, we not only unravel the mysteries of light’s interactions but also unlock its immense potential to shape our world.
Fluorescence and Phosphorescence: Illuminating the Night
In the captivating world of light, two enchanting phenomena emerge: fluorescence and phosphorescence. These mesmerizing effects transform ordinary objects into beacons of color, inviting us to explore their captivating nature.
Fluorescence: A Fleeting Dance of Light
Just as fireflies illuminate the night sky, fluorescence occurs when certain materials absorb light energy and promptly re-emit it in a cascade of vibrant hues. This brief burst of radiance lasts only as long as the excitation light remains present. Like a fleeting dance, the moment the light source vanishes, so does the fluorescence, leaving behind only a lingering memory of its magical presence.
Phosphorescence: A Glowing Legacy
Unlike its fleeting counterpart, phosphorescence leaves a more lasting impression. When exposed to light energy, phosphorescent materials absorb and store it within their molecular structure. This stored energy is slowly released over time, giving rise to a persistent glow that can endure for hours or even days after the excitation light has ceased. Think of it as a slow-motion ballet, where the captured light energy gracefully unwinds, releasing its ethereal glow into the darkness.
Distinguishing the Two: A Matter of Time
While both phenomena involve the emission of light, their temporal characteristics serve as their defining distinction. Fluorescence is an immediate response to excitation light, ceasing promptly when the light is removed. Phosphorescence, on the other hand, persists long after the excitation source is gone. This difference in duration arises from the different energy storage mechanisms employed by these materials.
Practical Applications: From Medical Diagnostics to Night Vision
Fluorescence and phosphorescence find widespread applications across various fields. Fluorescent dyes illuminate biological tissues in medical diagnostics, allowing doctors to visualize and analyze internal structures. Phosphorescent pigments enhance night vision devices, enabling soldiers and explorers to navigate in low-light conditions. These phenomena are also harnessed in security features, clothing, and artistic displays, adding a touch of enchantment to our everyday lives.
Threshold Frequency and the Photoelectric Effect
Imagine a scenario where light interacts with matter in a way that releases electrons. This phenomenon, known as the photoelectric effect, requires a specific condition to occur: the threshold frequency.
The threshold frequency is a critical limit for light to induce the photoelectric effect. Below this frequency, nothing happens. However, as the frequency of the light increases beyond this threshold, electrons are forcefully liberated from the material.
The photoelectric effect has been a topic of intense study since Albert Einstein proposed his groundbreaking equation explaining the phenomenon in 1905. Einstein’s equation connects the threshold frequency of a material to the work function of that material.
Work function represents the energy required to remove an electron from a material. Higher work functions indicate a stronger bond between the material and its electrons. Consequently, higher frequencies of light are necessary to overcome this bond and produce the photoelectric effect.
Einstein’s equation provides a precise mathematical relationship between threshold frequency and work function:
hf = W
Where:
- h is Planck’s constant
- f is the threshold frequency
- W is the work function
This equation has revolutionized our understanding of light-matter interactions and laid the foundation for quantum theory. By understanding threshold frequency and its role in the photoelectric effect, we have opened doors to new technologies like photomultipliers, light detectors, and even solar cells.
Unveiling the Enigmatic Work Function: A Key to Unlocking the Photoelectric Effect
As we delve deeper into the captivating world of light, we encounter a pivotal concept known as work function. This enigmatic property holds the key to understanding the photoelectric effect, a phenomenon that has revolutionized our comprehension of light-matter interactions.
Imagine a material’s surface as a picket fence, with electrons poised like intrepid adventurers eager to jump the fence. The work function represents the minimum amount of energy these electrons require to overcome the fence’s resistance and escape into the vast expanse of freedom. This energy is expressed in electron volts (eV), a unit that quantifies the energy of electrons.
The threshold frequency of a material, on the other hand, is the minimum frequency of light that can provide enough energy to enable electrons to surmount the work function barrier. Materials with higher work functions require higher threshold frequencies, meaning they are less likely to emit electrons under the influence of light.
The inextricable link between work function and threshold frequency is elegantly captured in Einstein’s equation for threshold frequency:
hf = W + K
where:
- h is Planck’s constant
- f is the threshold frequency
- W is the work function
- K is the kinetic energy of the emitted electron
This equation provides a crucial tool for calculating threshold frequencies and understanding the behavior of materials under the influence of light.
The work function of a material is influenced by its atomic structure and chemical composition. Metals generally have low work functions, making them more prone to emitting electrons under the influence of light. Non-metals, on the other hand, possess higher work functions, making them less likely to release electrons.
Understanding work function is essential for harnessing the photoelectric effect in practical applications. For instance, photocathodes in vacuum tubes rely on materials with low work functions to facilitate the emission of electrons under the influence of light. Solar cells, on the other hand, utilize materials with higher work functions to maximize the conversion of light energy into electrical energy.
As we unravel the mysteries of light-matter interactions, the work function emerges as a captivating and enigmatic property that unlocks the profound secrets of the photoelectric effect. By comprehending this concept, we empower ourselves to harness the power of light and forge transformative technologies that shape our world.
Einstein’s Equation for Threshold Frequency
- State Einstein’s equation and explain how it can be used to calculate threshold frequency
- Describe the significance of Einstein’s equation and its applications
Einstein’s Equation for Threshold Frequency: A Window into the Quantum World
In the realm of light and matter, the threshold frequency holds a pivotal place. This enigmatic concept is the gatekeeper of a fascinating phenomenon known as the photoelectric effect. Understanding the threshold frequency and its intricate relationship with light’s behavior is crucial for unraveling the mysteries of the quantum world.
Einstein’s Equation: A Guiding Light
In 1905, Albert Einstein revolutionized our understanding of light with his groundbreaking equation for the threshold frequency:
hf = φ + KE
where:
- h is Planck’s constant
- f is the frequency of the incident light
- φ is the work function of the material
- KE is the kinetic energy of the emitted electrons
This equation sheds light on the fundamental connection between the frequency of light and the energy it can transfer to electrons within a material. When light strikes a surface, it can excite electrons, causing them to break free from the material’s grip. However, this electron liberation only occurs if the light’s frequency surpasses a specific threshold value.
The Significance of Threshold Frequency
The threshold frequency serves as a boundary between two distinct realms: classical physics and quantum physics. In classical physics, light is perceived as a wave, but the photoelectric effect unveils its particle-like nature, known as photons. The threshold frequency represents the minimum frequency of light that carries enough energy to dislodge electrons from a given material.
Applications in the Real World
Einstein’s equation for threshold frequency has far-reaching applications in various fields. It underpins the operation of photomultipliers, which amplify faint light signals by converting photons into electrons. In semiconductor physics, the threshold frequency determines the energy bandgap of materials, governing their electrical and optical properties.