The Ph Scale: Measure Acid &Amp; Alkaline Strength &Amp; Balance
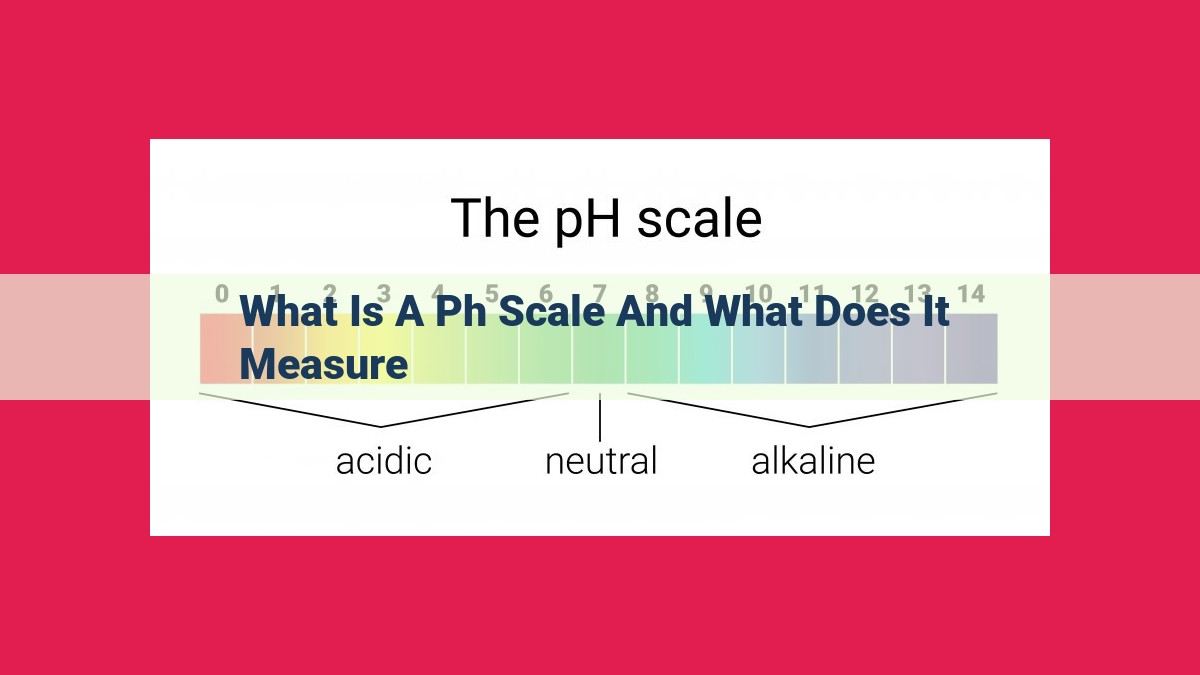
- The pH scale is a numerical measure of the acidity or alkalinity of a solution, ranging from 0 to 14.
- Acidity refers to solutions with a pH below 7, while alkalinity refers to solutions with a pH above 7.
- Neutral solutions have a pH of 7, indicating a balance between acidity and alkalinity.
Understanding the pH Scale: A Comprehensive Guide to Acidity and Alkalinity
In the realm of chemistry, one of the most fundamental concepts is the pH scale. It serves as a vital tool for measuring the acidity or alkalinity of a substance, providing valuable insights into its chemical nature and potential impact.
The pH scale is a logarithmic scale that ranges from 0 to 14. Acidity increases as the pH value decreases, while alkalinity increases as the pH value increases. A solution with a pH value of 7 is considered neutral, indicating an equal balance of acidity and alkalinity.
Acidity and the pH Scale
Acids are substances that release hydrogen ions (H+) when dissolved in water. The greater the concentration of hydrogen ions, the lower the pH value of the solution. Strong acids, such as hydrochloric acid and sulfuric acid, completely dissociate in water, releasing all of their hydrogen ions and resulting in a low pH value. Weak acids, like acetic acid, only partially dissociate, releasing a smaller number of hydrogen ions and producing a higher pH value.
Alkalinity and the pH Scale
Bases, on the other hand, release hydroxide ions (OH-) when dissolved in water. The higher the concentration of hydroxide ions, the higher the pH value of the solution. Strong bases, such as sodium hydroxide and potassium hydroxide, completely dissociate in water, releasing all of their hydroxide ions and resulting in a high pH value. Weak bases, like ammonia, only partially dissociate, releasing a smaller number of hydroxide ions and producing a lower pH value.
Acidity: The Essence of Acidity
Acidity, a fundamental concept in chemistry, refers to the degree to which a substance exhibits acid-like properties, often measured using the pH scale. This scale quantifies the acidity or alkalinity of a substance, with lower pH values indicating greater acidity.
Characteristics of Acidic Solutions
Acids are substances that produce hydrogen ions (H+) when dissolved in water. These H+ ions impart a sour taste, corrosive nature, and a pH below 7. Examples include hydrochloric acid (HCl) and sulfuric acid (H2SO4).
Strength of Acids
Acids vary in their strength, which depends on their tendency to dissociate in water. Strong acids completely dissociate, releasing all their H+ ions. Examples include HCl and H2SO4. Conversely, weak acids partially dissociate, releasing only a fraction of their H+ ions. Examples include acetic acid (CH3COOH) and carbonic acid (H2CO3).
Dissociation of Acids
When an acid dissolves in water, it releases hydrogen ions. For weak acids, this process is represented by the following equation:
HA + H2O <=> H3O+ + A-
where HA represents the acid, H3O+ represents the hydronium ion (which contributes to acidity), and A- represents the conjugate base of the acid. The stronger the acid, the more it dissociates, releasing more H+ ions and lowering the pH.
Alkalinity: The Balancing Force
In the realm of pH, there exists a force that counteracts acidity: alkalinity. It’s the gentle yet crucial counterpart that ensures a delicate equilibrium in our world.
Defining Alkalinity
Alkalinity refers to the ability of a substance to neutralize acids and raise the pH level. It’s measured on a scale of 0 to 14, with 7 being neutral. Substances with pH values above 7 are considered alkaline, or basic.
Properties of Basic Solutions
Basic solutions exhibit unique characteristics. They are typically:
- Slippery to the touch (due to the presence of hydroxide ions)
- Bitter in taste
- Able to conduct electricity (though not as efficiently as acids)
Strong and Weak Bases
Just like acids, bases can be classified as strong or weak. Strong bases dissociate completely in water, releasing a high concentration of hydroxide ions (OH-). Examples include sodium hydroxide (NaOH) and potassium hydroxide (KOH).
Weak bases dissociate only partially, forming an equilibrium between the undissociated base and hydroxide ions. Ammonia (NH3) is a well-known weak base.
Dissociation in Water
When a base dissolves in water, it undergoes dissociation. Strong bases dissociate completely, releasing all their hydroxide ions. Weak bases, on the other hand, dissociate only partially, creating a mixture of undissociated base and hydroxide ions.
This dissociation plays a crucial role in determining the pH value of a basic solution. The higher the concentration of hydroxide ions, the higher the pH value and the stronger the base.
pH Value: The Numerical Measure of Acidity and Basicity
In the realm of chemistry, where substances dance and interact, the pH value emerges as a crucial concept. It’s like a numerical compass that guides us through the vast ocean of acidity and alkalinity. But what exactly is pH value?
Simply put, pH value is a measure of the relative acidity or alkalinity of a solution. It’s a logarithmic scale that ranges from 0 to 14, with 7 representing neutrality. Acids have pH values below 7, while bases (alkaline solutions) have pH values above 7.
Calculating pH value is a straightforward process that involves measuring the concentration of hydrogen ions (H+) in a solution. The lower the concentration of H+, the higher the pH value. Conversely, the higher the concentration of H+, the lower the pH value.
The Significance of pH Values
pH values play a vital role in understanding the chemical behavior of solutions. They determine whether a substance is corrosive, reactive, or safe to handle. For example, highly acidic solutions (pH values below 2) can damage materials and cause skin irritation, while highly basic solutions (pH values above 12) can be caustic and harmful to living organisms.
In biological systems, pH values are crucial for maintaining healthy conditions. The human body, for instance, operates at a neutral pH of 7.4. Deviations from this pH range can lead to health problems, such as acidosis (low pH) or alkalosis (high pH).
The pH value is an indispensable tool for understanding the chemical nature of solutions. It allows us to quantify acidity and alkalinity, predict chemical behavior, and ensure the health and safety of our surroundings. By embracing the power of pH value, we unlock a deeper appreciation for the intricate chemical world that surrounds us.
Neutral Solutions: A Delicate Equilibrium
In the realm of chemistry, where acids and bases dance in an intricate tango, there exists a quiet sanctuary known as neutral solutions. These solutions occupy the middle ground, where the scales of acidity and alkalinity strike a harmonious balance.
Neutral solutions are characterized by a pH value of 7. This numerical indicator, measured on a scale from 0 to 14, represents the concentration of hydrogen ions (H+) in a solution. In neutral solutions, the concentration of H+ ions is precisely balanced by the concentration of hydroxide ions (OH-).
The pH scale, with neutral solutions perched at its midpoint, serves as a roadmap for understanding the chemical makeup of solutions. Solutions with pH values below 7 are considered acidic due to their higher concentration of H+ ions, while solutions with pH values above 7 are basic or alkaline due to their higher concentration of OH- ions.
Neutral solutions, with their equal distribution of H+ and OH- ions, form the foundation for life as we know it. Most biological processes, including those within our own bodies, thrive within a narrow range of pH values close to 7. Deviations from this delicate equilibrium can disrupt cellular functions, leading to potential health issues.
Understanding neutral solutions empowers us to appreciate the intricate balance that sustains life on Earth. It also highlights the importance of maintaining pH levels in various environments, ranging from our bodies to the world around us. By preserving this delicate equilibrium, we ensure the harmony of nature and the well-being of all living organisms.
Acidic Solutions: Lower pH Levels
Imagine a world where the balance of acidity and alkalinity is disrupted, leading to an acidic environment. These solutions, characterized by a low pH, possess distinct properties that can have a profound impact on materials and living organisms.
Characteristics of Acidic Solutions
Acidity is a fundamental property of substances that donate hydrogen ions (H+). Acidic solutions have a pH value below 7 on the pH scale. They are often sour or pungent in taste and can corrode metals. Additionally, high acidity levels can decompose organic matter and promote certain chemical reactions.
Implications of High Acidity
The effects of high acidity can be far-reaching. In industrial settings, acidic solutions can lead to equipment corrosion, necessitating costly maintenance and repairs. In the natural world, acidic rain can damage plant life, disrupt aquatic ecosystems, and even erode buildings.
Effects on Materials and Organisms
Acidic solutions can react with and weaken metal structures, such as steel and aluminum. Over time, this can lead to structural failures and safety concerns. Similarly, acidity can harm living organisms by damaging tissues, proteins, and other vital components. High levels of acidity can be lethal to some species and can disrupt the delicate balance of ecosystems.
Examples of Acidic Solutions
Common examples of acidic solutions include:
- Vinegar (pH around 2-3)
- Lemon juice (pH around 2-3)
- Battery acid (pH below 1)
- Hydrochloric acid (pH around 1-2)
Understanding the properties and implications of acidic solutions is crucial for industries, environmentalists, and scientists alike. By recognizing the potential risks and taking appropriate measures, we can mitigate the negative effects of high acidity and ensure the safety and well-being of our surroundings.
Basic Solutions: Understanding Their Properties and Hazards
What Makes a Solution Basic?
In the realm of chemistry, acidity and alkalinity are two ends of a spectrum. Basic solutions, often referred to as alkalis, possess a higher pH level than 7, indicating their alkaline nature. This characteristic stems from the presence of hydroxide ions (OH-) in the solution, which contribute to its alkalinity.
Properties of Basic Solutions
Basic solutions exhibit a range of distinct properties. They are slippery and bitter to the taste, a sensation attributed to their ability to react with the acids present in skin and saliva. Additionally, basic solutions turn red litmus paper blue and conduct electricity, further highlighting their unique chemical composition.
Dangers of High Alkalinity
While some basic solutions, such as household cleaners, are relatively mild, others can be highly corrosive. Exposure to strong alkalis can cause severe burns, eye damage, and respiratory problems. Their corrosive nature results from their ability to dissolve organic matter, including skin, tissue, and even metals.
Impact on Materials and Organisms
High alkalinity can also have deleterious effects on materials and living organisms. Alkaline solutions can damage fabrics, discolor metals, and weaken concrete. Similarly, exposure to high alkalinity can harm plants and animals, causing tissue damage, stunted growth, and even death.
Understanding the properties and hazards of basic solutions is crucial for safe handling and storage. Whether in the laboratory or in everyday applications, knowing the potential risks associated with alkalinity can help prevent accidents and protect both human health and the environment.
Strong Acids and Bases: A Force to Be Reckoned With
In the realm of chemistry, there exist two powerful entities: strong acids and strong bases. These substances exhibit extraordinary behavior in water, setting them apart from their weaker counterparts. Let’s delve into their captivating properties and unravel the implications of their immense strength.
Defining the Powerhouses: Strong Acids and Bases
Strong acids are chemical compounds that completely dissociate in water, releasing hydrogen ions (H+). This complete dissociation means that every molecule of the strong acid yields one or more H+ ions. Examples of strong acids include hydrochloric acid (HCl) and sulfuric acid (H2SO4).
Strong bases are the polar opposites of strong acids. They also dissociate completely in water, but they release hydroxide ions (OH-). These ions give strong bases their characteristic alkaline properties. Sodium hydroxide (NaOH) and potassium hydroxide (KOH) are prime examples of strong bases.
The Significance of Complete Dissociation
The complete dissociation of strong acids and bases has a profound impact on the pH values of the solutions they create. pH, a measure of acidity or alkalinity, is calculated on a scale from 0 to 14, with 0 being the most acidic and 14 being the most alkaline (or basic).
Strong acids and bases have extreme pH values. Strong acids have very low pH values, below 7, indicating their highly acidic nature. On the other hand, strong bases have very high pH values, above 7, highlighting their strong alkalinity.
The Implications of Strong Acids and Bases
The strength of acids and bases has significant implications in various scientific fields and everyday applications.
- Strong acids are highly corrosive and can cause severe damage to materials and living tissues. They are used in industrial processes such as metal refining and chemical manufacturing.
- Strong bases have a strong cleaning effect and are used in household cleaners, detergents, and industrial degreasers. However, they can also be caustic and cause skin irritation.
Strong acids and bases are powerful chemical compounds that play crucial roles in many areas of science and daily life. Their complete dissociation in water gives them unique properties and extreme pH values that distinguish them from their weaker counterparts. Understanding their characteristics and implications is essential for handling and utilizing these substances safely and effectively.
Weak Acids and Bases: A Milder Influence
In the realm of acidity and alkalinity, weak acids and bases play a crucial role in maintaining the delicate balance of chemical reactions. Unlike their more potent counterparts, strong acids and bases, weak acids and bases exhibit a milder influence on the pH scale, making them essential in countless applications.
Defining Weak Acids and Bases
Weak acids and bases possess a unique characteristic that sets them apart: partial dissociation. When they dissolve in water, they do not completely dissociate into ions. Instead, they form a partial equilibrium between the undissociated form and the ions, resulting in a less acidic or alkaline solution.
Partial Dissociation: A Dance of Equilibrium
The equilibrium established by weak acids and bases stems from their partial dissociation. Only a fraction of the acid or base molecules release ions into the solution, leaving a significant portion in their undissociated form. This equilibrium keeps the pH of the solution within a milder range.
Effect on pH Values
The partial dissociation of weak acids and bases has a profound effect on the pH values of the solutions they create. Weak acids produce solutions with pH values above 7 (neutral), while weak bases result in pH values below 7. This milder acidity or alkalinity makes them suitable for various applications, including buffer solutions and the regulation of pH levels in biological systems.
Weak acids and bases, with their unique behavior in water, play a vital role in shaping the chemical landscape of our world. Their partial dissociation allows for more precise control over pH levels, making them indispensable tools in fields ranging from chemistry and biology to environmental sciences and industry. Understanding their properties and applications is essential for navigating the intricate world of acidity and alkalinity.