The Nucleus: Unlocking The Secrets Of Atomic Identity And Chemical Properties
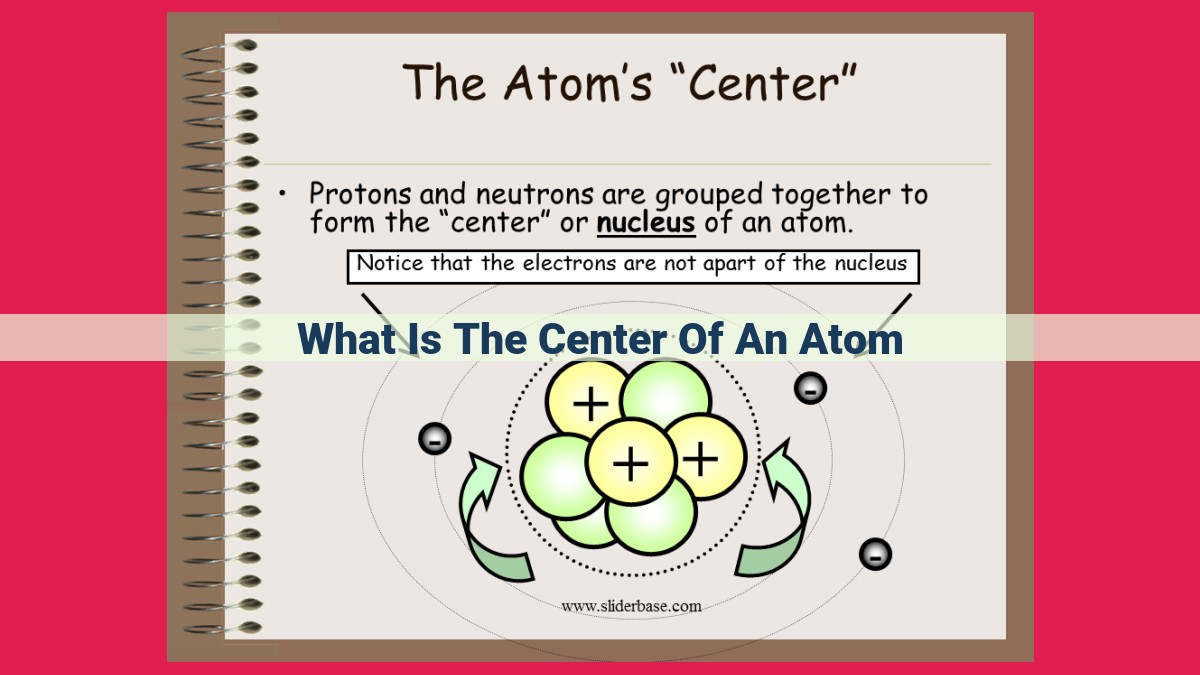
At the heart of every atom lies the nucleus, a dense, positively charged region that houses the atom’s identity. Composed of protons and neutrons, the nucleus determines an element’s atomic number and mass. Protons define the element’s type and provide its mass, while neutrons contribute to stability. Isotopes, variations of the same element with different neutron counts, alter the atomic mass but not the element’s identity. The atomic number and mass number, together with the number of valence electrons orbiting beyond the nucleus, shape an element’s unique chemical properties and position in the periodic table.
Unveiling the Heart of Matter: The Nucleus, Atom’s Core
- Definition and composition of the atomic nucleus, including protons and neutrons
Unveiling the Heart of Matter: The Nucleus, Atom’s Core
At the heart of every atom lies a tiny, dense, and incredibly powerful realm: the nucleus. This enigmatic core holds the secrets to an element’s identity, stability, and unique properties. Let’s delve into the fascinating world of the atomic nucleus, uncovering its composition and unraveling the mysteries it contains.
The Nucleus: A Hallowed Ground for Subatomic Particles
The atomic nucleus resides at the center of the atom, occupying a space thousands of times smaller than the atom itself. Within this microscopic realm, two fundamental particles reside: protons and neutrons.
Protons: The Guardians of Identity and Mass
Protons are positively charged particles that define an element’s identity. Each element possesses a unique atomic number, which corresponds to the number of protons in its nucleus. This atomic number dictates an element’s position on the periodic table and its fundamental chemical properties.
In addition to their role in defining identity, protons contribute to the mass of the atom. Together with neutrons, protons determine an atom’s mass number, which represents the total number of protons and neutrons in the nucleus.
Neutrons: The Unsung Heroes of Stability
While protons hold the key to an element’s identity, neutrons play a crucial role in stabilizing the nucleus. Neutrons are uncharged particles that neutralize the positive charge of protons and provide a delicate balance within the atomic core.
Just like protons, neutrons contribute to the mass of the atom, but they do not alter its atomic number. This interplay of protons and neutrons gives rise to isotopes, atoms of the same element that differ in neutron count and, thus, mass.
Atomic Number and the Periodic Table’s Orderly Dance
The atomic number of an element determines its position in the periodic table. Elements are arranged horizontally according to their increasing atomic numbers, creating a cohesive symphony of chemical properties. This orderly arrangement reveals patterns and trends that govern the behavior of elements.
Mass Number and the Rich Tapestry of Isotopes
The mass number of an element reflects the combined number of protons and neutrons in its nucleus. While most elements occur as a single stable isotope, some possess multiple isotopes with varying neutron counts. These isotopic variations contribute to the diverse atomic masses observed in nature.
Beyond the Nucleus: Valence Electrons Take the Stage
Our journey through the atomic nucleus would be incomplete without a glimpse at valence electrons. These electrons reside outside the nucleus in specific energy levels, and their arrangement dictates an atom’s reactivity and ability to form chemical bonds.
Valence electrons determine an element’s place in the periodic table and influence its chemical properties. They are the gateway to understanding chemical reactions and the intricate tapestry of molecular interactions.
Protons: Defining the Element and Mass
- Explain the concept of atomic number and how it relates to an element’s identity
- Discuss the role of protons in determining atomic mass
Protons: The Identity and Mass of Atoms
Within the microscopic realm of atoms, the nucleus stands as the heart, housing the fundamental building blocks that define each element and bestow its unique properties. Among these building blocks, protons play a pivotal role in determining an element’s identity and its atomic mass.
The atomic number of an element is the number of protons residing within its nucleus. This number is unique to each element and serves as the defining characteristic that distinguishes one element from another. For instance, an atom of hydrogen has one proton, while an atom of carbon possesses six protons.
Protons also significantly influence an atom’s atomic mass, which is the weighted average of the masses of all protons, neutrons, and electrons in the atom. As protons possess a relatively large mass compared to electrons, they contribute the most to an atom’s overall mass. The atomic mass of an element is expressed in atomic mass units (amu), and it represents the average mass of all isotopes of that element.
Isotopes are variations of the same element that have the same number of protons but differ in the number of neutrons. While isotopes do not alter an element’s identity or atomic number, they can affect its atomic mass. For example, carbon has three isotopes: carbon-12, carbon-13, and carbon-14. All three isotopes have six protons but varying numbers of neutrons, resulting in different atomic masses.
Neutrons: The Silent Guardians of Nuclear Stability
In the heart of every atom lies a tightly packed core known as the nucleus, where protons and neutrons reside in harmonious balance. While protons define an element’s identity, neutrons play a crucial, yet often overlooked, role in maintaining nuclear stability.
Isotopes: Unveiling the Nuclear Diversity
Atoms of the same element can come in slightly different forms called isotopes. Isotopes share the same atomic number (number of protons), but vary in the number of neutrons they harbor. This variation in neutron count subtly alters an atom’s atomic mass without changing its chemical properties. For example, carbon-12 has six protons and six neutrons, while carbon-14 has six protons and eight neutrons.
The Balancing Act of Neutrons
Neutrons act as a stabilizing force within the nucleus. Protons carry a positive charge, repelling each other. To counteract this electrostatic repulsion, neutrons, which are electrically neutral, provide a calming effect. They act as a buffer between protons, reducing their tendency to fly apart.
In certain isotopes, the number of neutrons is critical for stabilizing the nucleus. For instance, atomic nuclei with an odd number of protons require an even number of neutrons to maintain stability. This is known as the odd-even rule. Conversely, nuclei with an even number of protons favor an odd number of neutrons.
Stability’s Silent Heroes
The role of neutrons in nuclear stability is often understated, but their presence is essential for the integrity of matter as we know it. Without neutrons, the repulsive forces between protons would tear atoms apart, rendering life as we know it impossible.
So, let us give credit where it’s due. While protons define an element’s identity, it’s the silent guardians, the unassuming heroes, the neutrons that ensure the stability of the atomic nucleus. They may not be as glamorous as protons, but they are indispensable in maintaining the harmony of the universe.
Atomic Number and Element Classification: Unraveling the Periodic Puzzle
The atomic nucleus, the heart of every atom, holds the key to understanding the arrangement of elements in the periodic table, a map that reveals the unique properties of each element. Atomic number, a fundamental характеристика of an atom, plays a pivotal role in determining an element’s identity and its place in this organizational system.
The atomic number of an element is defined by the number of protons in its nucleus. Protons carry a positive charge and contribute to the mass of the atom. Each element has a unique atomic number, which differentiates it from all other elements. For instance, hydrogen has an atomic number of 1, indicating the presence of a single proton in its nucleus; helium has an atomic number of 2, signifying two protons in its nucleus; and so on.
The atomic number establishes the element’s position in the periodic table. Elements are arranged horizontally in rows (periods) and vertically in columns (groups) based on their atomic number. Elements in the same group share similar chemical properties due to having the same number of valence electrons, which are responsible for chemical bonding.
The periodic table serves as a powerful tool for predicting an element’s properties. By identifying an element’s atomic number, scientists can infer its position in the table and thus gain insights into its reactivity, oxidation state, electronegativity, and other essential characteristics. It’s a testament to the profound importance of atomic number in shaping the chemical world, allowing us to understand and predict the behavior of the elements that make up our universe.
Mass Number and Atomic Mass: Unveiling the Essence of an Atom
At the heart of every atom lies a tiny, dense core called the nucleus. Within this minute realm, the secrets of an element’s identity and mass are held. One of the fundamental concepts to unravel this atomic mystery is mass number.
The mass number, denoted by the symbol A, represents the total number of subatomic particles within the nucleus. These particles, protons and neutrons, contribute equally to the atom’s mass. Protons carry a positive charge and determine the element’s identity, while neutrons carry no charge and provide stability to the nucleus.
However, the atomic mass of an element, expressed in atomic mass units (amu), is not an exact whole number. This is because, for many elements, multiple isotopes exist. Isotopes are variations of the same element that have the same number of protons but differ in the number of neutrons.
Consider the element carbon, for instance. The most common isotope, carbon-12, has 6 protons and 6 neutrons, giving it a mass number of 12. However, there are also isotopes of carbon with 7 and 8 neutrons, known as carbon-13 and carbon-14, respectively.
The atomic mass reported for carbon is an average of the masses of all its isotopes, weighted by their natural abundance. This average value takes into account the contributions of all the protons and neutrons in each isotope, providing a more precise representation of the element’s overall mass.
Understanding mass number and atomic mass is crucial for unraveling the complexities of the atomic world. These concepts pave the way for a deeper exploration of the properties and behavior of elements, enabling us to better comprehend the fundamental building blocks of our universe.
Beyond the Nucleus: Unlocking the Secrets of Valence Electrons
As we delve into the inner workings of atoms, we discover the fascinating world beyond the nucleus, where valence electrons play a pivotal role in shaping matter’s behavior. These electrons, located in the outermost shells of atoms, are the key players in chemical bonding and determine an element’s reactivity.
Valence Electrons: On the Frontier of Reactivity
Envision valence electrons as the bridge between atoms, enabling them to interact and form molecules. The number of valence electrons an atom possesses dictates its bonding potential. Atoms with numerous valence electrons tend to be highly reactive, eagerly sharing or exchanging electrons to achieve a stable configuration.
A Dance of Attraction: Chemical Bonding through Valence Electrons
The dance of valence electrons orchestrates the formation of chemical bonds. When atoms come close enough, their valence electrons can overlap, creating a shared space of electrons. This shared electron cloud attracts the positively charged nuclei of both atoms, holding them together in a stable bond.
Reactivity: A Symphony of Valence Electrons
The number of valence electrons also governs an element’s reactivity. Elements with a full valence shell, such as noble gases, tend to be inert, as they have no need to gain or lose electrons to achieve stability. However, elements with incomplete valence shells are highly reactive, eager to acquire or shed electrons to complete their outermost shell.
Valence electrons are the unsung heroes of chemistry, dictating the bonding behavior and reactivity of elements. Their understanding is crucial for unraveling the intricate tapestry of chemical reactions and the properties of the materials that shape our world.