The Nitrogen Cycle: Essential For Life, Climate Regulation, And Plant Growth
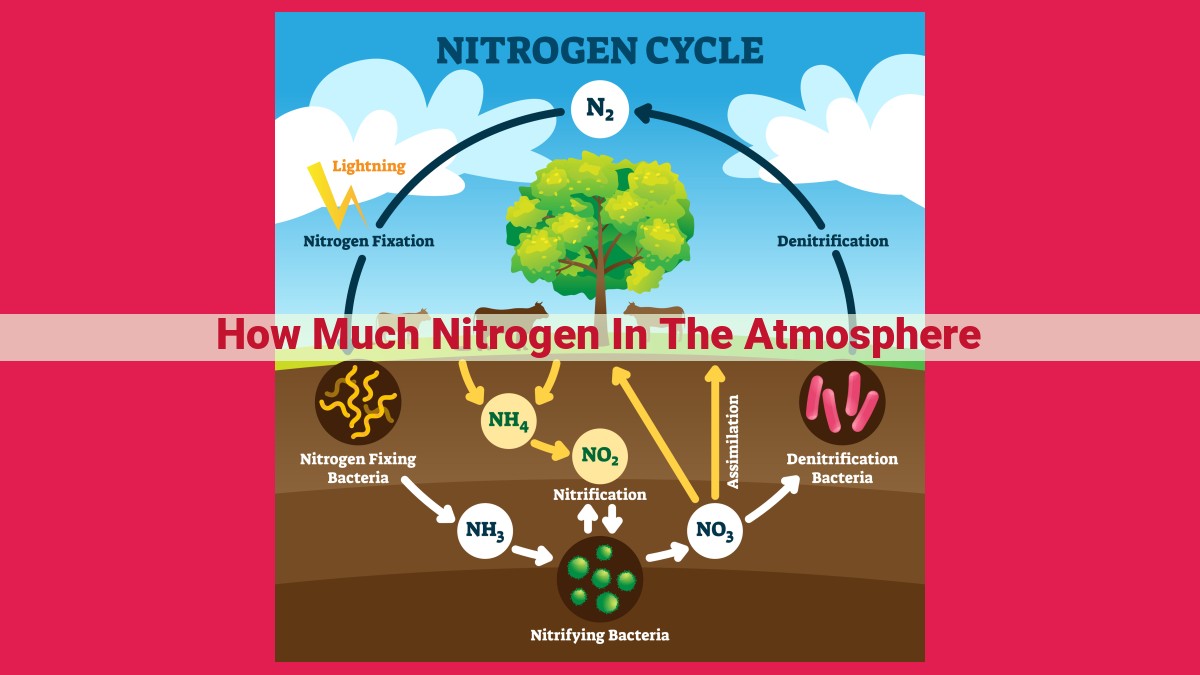
Nitrogen, comprising approximately 78% of Earth’s atmosphere, is essential for life. Through the nitrogen cycle, bacteria transform atmospheric nitrogen into usable compounds, enabling plant growth. Denitrification returns nitrogen to the atmosphere, regulating its availability. Nitrogen compounds are vital plant nutrients, and their release during the nitrogen cycle produces nitrous oxide, a greenhouse gas contributing to climate change.
The Nitrogen Cycle: The Key to Nitrogen Abundance
- Explain the interconnected processes of nitrogen fixation, denitrification, and their role in maintaining nitrogen levels.
The Nitrogen Cycle: The Key to Nitrogen Abundance
Nitrogen is the most abundant element in Earth’s atmosphere, yet it is ironically scarce in forms that plants and animals can use. Thankfully, nature has devised an intricate dance, known as the Nitrogen Cycle, that makes this essential nutrient available to life.
This captivating cycle revolves around three interconnected processes: nitrogen fixation, denitrification, and atmospheric nitrogen. Let’s dive into each, uncovering their significance in maintaining nitrogen levels.
Nitrogen Fixation: The Birth of Nitrogen Compounds
Nitrogen fixation is the miraculous ability of certain bacteria to convert atmospheric nitrogen into usable forms. These nitrogen-fixing bacteria possess a unique enzyme called nitrogenase, which breaks down the strong bonds that hold nitrogen atoms together.
Once separated, nitrogen atoms can combine with other elements to form ammonia (NH3), the simplest nitrogen compound. Ammonia is then further processed by plants and other organisms, ultimately enriching the soil and supporting plant growth.
Denitrification: Reclaiming Nitrogen from Soil and Water
Denitrification is the mirror image of nitrogen fixation. It converts nitrates (NO3-) and nitrites (NO2-) back into atmospheric nitrogen. This process is carried out by a different group of bacteria that thrive in low-oxygen environments, such as waterlogged soils or wetlands.
Denitrification ensures that nitrogen is not lost from the ecosystem and is continually recycled. It helps regulate nitrogen availability, preventing excessive accumulation that could harm plants and contribute to water pollution.
Atmospheric Nitrogen: The Earth’s Nitrogen Reservoir
The Earth’s atmosphere is a vast reservoir of nitrogen, accounting for 78% of its volume. Atmospheric Nitrogen (N2) is a highly inert gas, meaning its atoms are tightly bonded and not easily reactive. However, during nitrogen fixation, a small portion of atmospheric nitrogen is converted into usable forms, fueling the nitrogen cycle.
Nitrogen Fixation: The Birth of Nitrogen Compounds
- Describe the unique ability of certain bacteria to convert atmospheric nitrogen into usable forms, enabling plant growth.
Nitrogen Fixation: The Birth of Nitrogen Compounds
Nitrogen is essential for life, a building block for proteins, nucleic acids, and chlorophyll. However, most organisms cannot utilize nitrogen in its atmospheric form. Enter nitrogen-fixing bacteria, the unsung heroes that transform atmospheric nitrogen into usable forms, enabling the growth of plants and the flourishing of life.
These remarkable bacteria possess the unique ability to convert inert nitrogen gas into ammonia, a form plants can absorb. The process, known as nitrogen fixation, occurs in two main ways: biological and industrial.
Biological Nitrogen Fixation: This marvel is performed by certain bacteria, such as Rhizobium and Bradyrhizobium. These bacteria form symbiotic relationships with legumes like peas, beans, and clover. They reside in nodules on the plant’s roots, where they convert atmospheric nitrogen into ammonia. The plant, in turn, provides the bacteria with energy and shelter.
Industrial Nitrogen Fixation: Humans have also developed ways to fix nitrogen on a large scale through a process called the Haber-Bosch process. This industrial method involves combining nitrogen and hydrogen under high pressure and temperature to produce ammonia.
The importance of nitrogen fixation cannot be overstated. By converting atmospheric nitrogen into usable forms, nitrogen-fixing bacteria make nitrogen available to plants. Plants then use this nitrogen to create proteins, chlorophyll, and other essential compounds. Without nitrogen-fixing bacteria, the cycle of life as we know it would not be possible.
So, next time you bite into a juicy tomato or admire a field of sunflowers, remember the unsung heroes beneath the soil – nitrogen-fixing bacteria – who make these life-sustaining marvels possible.
Denitrification: Reclaiming Nitrogen from Soil and Water
In the intricate tapestry of the nitrogen cycle, denitrification plays a pivotal role, meticulously unwinding the intricate threads of nitrogen compounds to replenish the atmospheric reservoir. This process, orchestrated by a select group of anaerobic bacteria, is essential for maintaining a delicate balance in nature’s nitrogen budget, ensuring its continued availability for plant growth and ecosystem health.
Imagine a subterranean labyrinth where nitrates, once the building blocks of plant proteins, undergo a remarkable transformation. In these oxygen-depleted depths, denitrifying bacteria emerge as the masterminds, employing a unique enzymatic prowess to break down nitrates into simpler forms. Through a series of intricate chemical reactions, nitrates are converted into nitrites, nitric oxide, and ultimately dinitrogen gas.
This remarkable feat of denitrification serves as a safety valve, preventing the accumulation of excessive nitrogen in soil and water, which can lead to ecosystem imbalances and water pollution. By returning nitrogen to its atmospheric form, denitrification safeguards against the detrimental effects of nitrogen overload, ensuring its availability for future generations of life.
Atmospheric Nitrogen: The Earth’s Limitless Nitrogen Reservoir
Nitrogen, the most abundant element in our atmosphere, holds the key to sustaining life on Earth. This invisible ocean of nitrogen serves as the primary source for the nitrogen cycle, the intricate dance of transformations that ensures nitrogen’s availability for plants and microorganisms.
Vast and Dynamic
The Earth’s atmosphere is a colossal nitrogen reservoir, holding an immense 78% of this gas. This nitrogen pool is remarkably stable, maintained by a balance between nitrogen’s release and reabsorption. However, this tranquillity is not static; atmospheric nitrogen is a vibrant participant in the nitrogen cycle.
The Source of All Nitrogen
Atmospheric nitrogen serves as the ultimate source for the nitrogenous compounds that are essential for life. Nitrogen-fixing bacteria possess the extraordinary ability to convert atmospheric nitrogen into ammonia, the first step in the nitrogen cycle. This transformation, known as nitrogen fixation, unlocks the nitrogen reservoir, making it accessible to other organisms.
The Importance of Atmospheric Nitrogen
The nitrogen in our atmosphere is not only abundant but also critical. It is the foundation for all nitrogen compounds necessary for plant growth, such as ammonia, nitrates, and nitrites. These compounds provide the nitrogen building blocks for proteins, nucleic acids, and other essential biomolecules.
The Earth’s atmosphere is not merely a passive storehouse of nitrogen but an active player in the nitrogen cycle. Its vast nitrogen reservoir is the lifeline that sustains the vitality of our planet. By understanding the role of atmospheric nitrogen, we gain a deeper appreciation for the interconnectedness of life and the delicate balance that supports it.
Nitrogen Compounds: The Essential Nutrients for Plant Health
In the vast tapestry of life’s processes, the nitrogen cycle plays a pivotal role, providing the essential building blocks for life on Earth. Among the myriad compounds involved in this intricate cycle, nitrogen in its diverse forms holds a special significance, nourishing the verdant realm of plants and shaping the delicate balance of our ecosystems.
In the plant kingdom, nitrogen serves as the cornerstone of life’s symphony. Essential for chlorophyll production, the green pigment that harnesses light’s energy for photosynthesis, nitrogen compounds also play a vital role in protein synthesis, the very fabric of life itself.
Ammonia: The Building Block of Life
In its simplest form, nitrogen exists as ammonia, a pungent gas that forms the foundation for nitrogen assimilation in plants. This colorless compound, released by bacteria during decomposition, is quickly absorbed by plant roots and converted into more complex nitrogen compounds.
Nitrates: The Powerhouse of Nutrition
Nitrates, the most abundant form of nitrogen in soil, are the preferred source of nitrogen for most plants. Readily taken up by roots, these negatively charged ions are transported throughout the plant, providing energy for growth and development.
Nitrites: The Middle Ground
Nitrites, the intermediate form between ammonia and nitrates, also contribute to plant nutrition. However, their presence is often transient, as they are quickly converted to nitrates by beneficial soil bacteria.
The interplay of these nitrogen compounds is crucial for plant health. Deficiencies can lead to stunted growth, yellowing leaves, and reduced yields. Conversely, excessive nitrogen levels can promote excessive vegetative growth at the expense of fruit or seed production.
Understanding the role of nitrogen compounds in plant nutrition is essential for fostering healthy and productive plant ecosystems. By providing the right balance of nitrogen forms, we can unlock the full potential of plants, ensuring a vibrant and sustainable future for our planet.
Nitrous Oxide: A Greenhouse Gas with a Hidden Impact
- Discuss the release of nitrous oxide during nitrogen transformations, its contribution to climate change, and its long-term atmospheric presence.
Nitrous Oxide: A Hidden Greenhouse Gas with a Surprising Source
The nitrogen cycle is a natural process that maintains the balance of nitrogen in our ecosystems. It involves the conversion of atmospheric nitrogen into usable forms for plants, which eventually return it to the atmosphere.
One step in this cycle, denitrification, is the process by which certain bacteria convert nitrates in soil and water back into atmospheric nitrogen. However, this process also releases a potent greenhouse gas: nitrous oxide.
Nitrous oxide has a long atmospheric lifetime and is 298 times more powerful than carbon dioxide in trapping heat. While its emissions are lower than other greenhouse gases like carbon dioxide, its contribution to climate change is significant.
The release of nitrous oxide occurs during various nitrogen transformations. This includes the breakdown of nitrogen fertilizers in agricultural fields, the decomposition of organic matter in wetlands, and combustion processes in engines.
Reducing nitrous oxide emissions requires a multi-faceted approach. Improving nitrogen fertilizer management practices, promoting sustainable agricultural practices, and implementing advanced emission control technologies can help mitigate this hidden impact on our climate.
By understanding the role of nitrous oxide in the nitrogen cycle, we can take informed actions to reduce its emissions and contribute to a more sustainable future.