The Link Reaction: Unlocking Cellular Energy Through Water, Nadh, And Fadh2
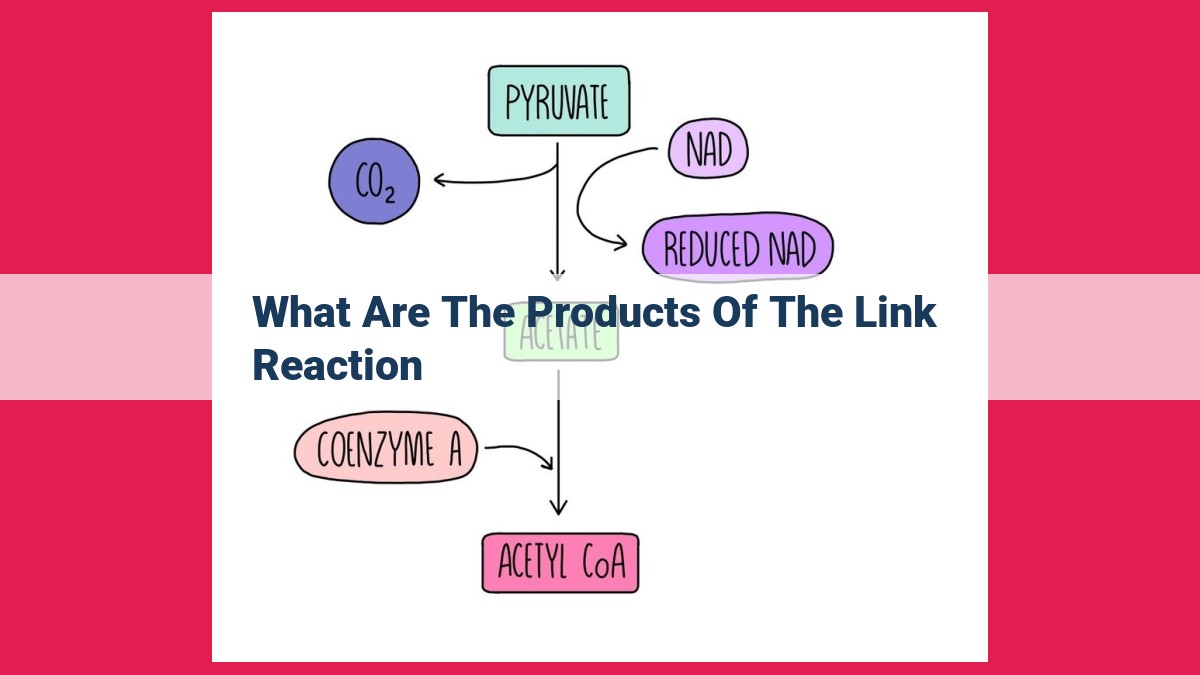
The link reaction, a crucial step in cellular respiration, produces three significant products: water, NADH, and FADH2. Water serves as a solvent and facilitates cellular processes. NADH and FADH2 are high-energy electron carriers, playing key roles in oxidative phosphorylation, the process that generates most of the cell’s energy. These products are essential for the continuation of cellular respiration, the breakdown of glucose to produce ATP, the primary energy currency of the cell.
The Link Reaction: A Crucial Step in Cellular Respiration
In the bustling city of our cells, energy is the lifeblood, and cellular respiration is the bustling metropolis that generates that vital energy. At the heart of this metropolis lies the link reaction, a pivotal step that connects two major pathways: glycolysis and the TCA cycle.
Like a skilled conductor, the link reaction orchestrates the transition from glycolysis, where glucose is broken down into pyruvate, to the TCA cycle, where pyruvate is further broken down and oxidized to produce carbon dioxide and ATP.
Water: A Silent Bystander with a Vital Role
As the link reaction unfolds, a byproduct quietly emerges: water. Often overlooked, water plays a crucial role as a universal solvent in cellular processes. It facilitates the transportation of nutrients, waste removal, and temperature regulation, ensuring the smooth operation of our cellular machinery.
NADH and FADH2: The Power Duo of Electron Carriers
The link reaction also generates two high-energy electron carriers: NADH and FADH2. These molecules are the workhorses of oxidative phosphorylation, a process that produces the bulk of our cellular energy.
NADH carries electrons from pyruvate to the electron transport chain, where they are used to pump hydrogen ions across a membrane, creating an electrochemical gradient that drives ATP synthesis.
FADH2, a less potent electron carrier, also feeds electrons into the electron transport chain, contributing to the energy-producing process.
Connecting the Dots: Relationships within Cellular Respiration
The link reaction is not an isolated event but is intricately connected to other aspects of cellular respiration. It involves oxidation-reduction reactions, where electrons are transferred between molecules, as well as hydrogen bonding, the weak but essential force that stabilizes water molecules and other biological structures.
By understanding the integral role of the link reaction and its products, we gain a deeper appreciation for the complexity and interconnectedness of cellular respiration, the powerhouse that fuels our cells and sustains life itself.
Water: The Serendipitous Byproduct of Cellular Energy Production
Within the intricate machinery of our cells lies a remarkable process known as cellular respiration, a dance of energy transformation that sustains our very existence. As part of this elaborate choreography, a critical juncture emerges: the link reaction, where chemical bonds are reshuffled and unexpected products are born.
One such byproduct, so ordinary in its presence yet profound in its significance, is water. Produced in copious quantities, water plays a pivotal role in the intricate tapestry of life. Its ability to dissolve a multitude of substances makes it a versatile solvent, facilitating countless cellular processes.
Like an invisible hand, water transports nutrients, enzymes, and waste products within our cells. It regulates temperature, preventing potentially damaging fluctuations. Its presence is essential for the proper functioning of proteins, the workhorses of cellular life. Without water’s nurturing touch, the symphony of cellular respiration would falter and cease.
Water’s role in the link reaction is an elegant reminder of nature’s interconnectedness. While the primary goal of cellular respiration is energy production, the simple byproduct of water serves a multitude of indispensable purposes. Like a hidden gem, it reveals the subtle beauty and complexity that underlies the very foundation of life.
NADH: The Powerhouse of Oxidative Phosphorylation
In the intricate dance of cellular respiration, the link reaction plays a pivotal role, producing NADH, a high-energy electron carrier that fuels the metabolic engine. This remarkable molecule is generated through a series of chemical events, each a testament to the incredible complexity and efficiency of biological processes.
The link reaction serves as a bridge between glycolysis and the Krebs cycle, two crucial stages of cellular respiration. During glycolysis, glucose, the cell’s primary energy source, is broken down into smaller molecules. This process releases energy, capturing it in NADH. The link reaction then transfers these electron-rich NADH molecules to the electron transport chain, where they serve as the driving force for oxidative phosphorylation.
Oxidative phosphorylation is the crowning achievement of cellular respiration. It generates the majority of the cell’s energy, harnessing the power of electron transfer. As NADH releases its electrons to the electron transport chain, its energy is captured as a proton gradient across the mitochondrial inner membrane. This gradient drives the synthesis of ATP, the universal currency of cellular energy.
NADH is not just an inert carrier of electrons; it is an essential participant in the redox reactions that characterize oxidative phosphorylation. Redox reactions involve the transfer of electrons between molecules, a process that underlies many biological reactions. NADH acts as an electron donor, providing the fuel for the electron transport chain to convert ADP into ATP.
In essence, NADH is the energy-storing powerhouse of oxidative phosphorylation. Its production in the link reaction marks the beginning of a molecular symphony that ultimately generates the ATP that powers all cellular activities. Without NADH, the cell would be devoid of energy, unable to perform the vital functions that sustain life.
FADH2: A Crucial Electron Carrier in Cellular Respiration
In the intricate world of cellular respiration, a series of chemical reactions orchestrate the conversion of glucose into energy-rich molecules. Among these reactions, the link reaction plays a pivotal role, producing water and electron carriers essential for the continuation of cellular life.
One of these electron carriers is FADH2, a molecule that holds a key to the next stage of cellular respiration: oxidative phosphorylation. As the link reaction unfolds, succinate dehydrogenase enzyme facilitates the transfer of electrons from succinate to FADH. This transfer results in the formation of FADH2, which then embarks on a journey through the electron transport chain to the final electron acceptor, oxygen.
Along this chain of protein complexes, FADH2 undergoes a series of redox reactions, donating its electrons to carrier molecules. As the electrons flow through the chain, their energy is harnessed to pump hydrogen ions across the inner mitochondrial membrane. This electrochemical gradient, a reservoir of potential energy, serves as the driving force for ATP synthesis, the molecule responsible for powering cellular activities.
FADH2, though often overshadowed by its more recognized counterpart NADH, plays an indispensable role in the electron transport chain. Its contribution to the overall process of oxidative phosphorylation underscores the intricate interplay of various electron carriers in the delicate dance of cellular energy production.
The Link Reaction: Connecting Elements of Cellular Respiration
In the intricate symphony of cellular respiration, the link reaction plays a pivotal role, orchestrating the conversion of energy-rich substrates into usable cellular currency. This vital process links glycolysis, the breakdown of glucose, to subsequent energy-generating reactions.
From the link reaction emerges a trio of key products: water, NADH, and FADH2. Each of these substances contributes uniquely to the energy-generating machinery that powers our cells.
Water: The Solvent of Life
As the link reaction proceeds, a molecule of water is released as a byproduct. This seemingly innocuous substance is far from inert. As the universal solvent, water facilitates myriad biochemical reactions, transporting vital molecules and ions throughout the cell.
NADH: The Electron Carrier
NADH, a high-energy electron carrier, is generated during the link reaction. This molecule donates its electrons to the electron transport chain, a series of proteins that shuttle electrons to create a proton gradient across the inner mitochondrial membrane. This gradient drives the synthesis of ATP, the primary energy currency of cells.
FADH2: A Supporting Electron Donor
FADH2, another electron carrier, is also produced in the link reaction. While it contributes fewer electrons to the electron transport chain than NADH, it nevertheless plays a supporting role in energy production.
The Interplay of Related Concepts
The link reaction and its products are intimately connected to other aspects of cellular respiration. The oxidation-reduction reactions involved in the link reaction transfer electrons to electron carriers, driving the electron transport chain.
Oxidative phosphorylation utilizes the proton gradient generated by the electron transport chain to synthesize ATP. This process is essential for maintaining cellular energy levels.
Hydrogen bonding stabilizes the water molecule produced in the link reaction, enabling it to fulfill its solvent role. This bonding also influences the structure and function of proteins involved in oxidative phosphorylation.
The link reaction is a crucial stage in cellular respiration, connecting the breakdown of glucose to the generation of ATP. Its products, water, NADH, and FADH2, play key roles in electron transport, oxidative phosphorylation, and the overall energy balance of cells. Understanding the link reaction provides a glimpse into the complex and interconnected world of cellular metabolism.