Unlock The Building Blocks Of Matter: A Comprehensive Guide To Atoms And Elements
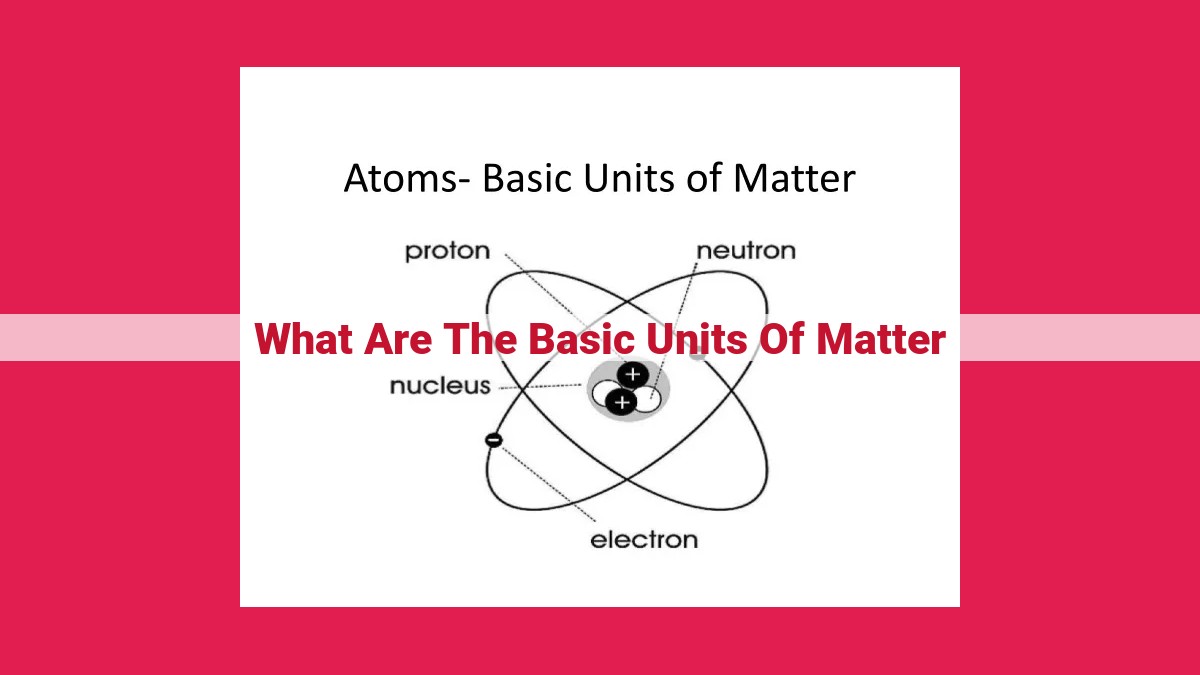
The basic units of matter are atoms, which are composed of even smaller subatomic particles—protons, neutrons, and electrons. Elements consist of only one type of atom, while compounds are combinations of different atoms that form molecules. Elements are organized systematically in the periodic table, which predicts their properties based on electron configuration. Atoms can lose or gain electrons to become ions, acquiring a positive or negative charge.
The Fundamental Building Blocks of Matter: Unveiling the Secrets of Our Universe
Matter, the essence of everything we see, touch, and feel, is a captivating realm of wonders. It’s a vibrant tapestry woven from the intricate dance of microscopic particles, each playing a pivotal role in shaping our existence.
At the heart of this mesmerizing tapestry lies the atom, the smallest unit of matter that retains the chemical properties of an element. Atoms are composed of an even tinier nucleus, which houses positively charged protons and neutral neutrons, surrounded by a cloud of negatively charged electrons.
To understand the diversity of matter around us, we must delve into the concept of elements. Elements are pure substances made up of atoms with the same number of protons. Each element occupies a unique position on the periodic table, a roadmap that organizes elements based on their atomic number and properties.
As atoms interact, they form compounds, substances composed of two or more different elements chemically bonded together. These bonds, such as covalent and ionic bonds, hold atoms together and give rise to the vast array of molecules that make up the world around us.
Atoms: The Indivisible Building Blocks of Matter
Our world, in all its complexity and diversity, is ultimately composed of tiny, indivisible units called atoms. These fundamental building blocks are the very essence of matter and the foundation upon which our understanding of the physical world is built.
Imagine the universe as an intricate tapestry, and atoms as the threads that weave it together. Inside each atom lies a dense, positively charged core known as the nucleus, surrounded by a swarm of negatively charged electrons. The nucleus is home to protons, particles with a positive charge, and neutrons, particles with no charge. The number of protons in the nucleus determines the atom’s identity, placing it on the periodic table of elements.
Electrons, on the other hand, orbit the nucleus in distinct energy levels, like miniature planets circling a star. These electrons are responsible for the atom’s chemical bonding abilities, allowing atoms to combine and form the vast array of molecules and compounds that make up everything around us.
By understanding the structure and properties of atoms, we unravel the secrets of matter and unlock the mysteries of the universe. Atoms are the fundamental building blocks upon which all science rests, and they hold the key to unlocking the wonders of our world.
Elements: The Foundations of the Periodic Table
- Define elements as substances composed of one atom type.
- Explain the atomic number and its significance in identifying elements.
- Discuss element classification in the periodic table.
Elements: The Cornerstones of the Periodic Table
In the intricate fabric of matter, elements serve as the fundamental building blocks, each possessing a unique identity and properties that shape our world. These elements are the indivisible entities that constitute the diverse substances we encounter in nature.
At the heart of each element lies its atomic number, a distinctive number that identifies the element on the periodic table. It represents the number of protons, positively charged particles, residing in the nucleus of each atom. The atomic number plays a crucial role in determining an element’s chemical behavior and place in the periodic table.
The periodic table, an ingenious organizational chart, arranges elements in a manner that reveals their similarities and trends. Elements are grouped into vertical columns, known as groups, based on their shared chemical properties. These properties arise from the number of valence electrons, the electrons in the outermost shell of an atom, which determine an element’s reactivity.
Across the table, elements are arranged in horizontal rows called periods. As you move from left to right across a period, the atomic number increases, resulting in a gradual change in properties. Elements in the same period have the same number of electron shells, giving them similar chemical behaviors.
By understanding the periodic table, scientists can predict the properties of elements based on their position. For example, elements in the same group often exhibit similar reactivity, while elements in the same period have similar physical properties. This organizational tool provides invaluable insights into the elemental world, enabling us to comprehend the complex interactions that govern chemical reactions and the formation of matter.
Compounds: The Interplay of Atoms and Chemical Bonds
In the realm of matter, atoms, the fundamental building blocks, don’t always play solo. They often collaborate to create compounds, substances composed of chemically bonded atoms. These compounds are the driving force behind countless phenomena in our world, from the air we breathe to the food we eat.
The Birth of Molecules: Atoms Joining Forces
When atoms come together to form compounds, they do so through chemical bonds. These bonds are the invisible glue that holds atoms together, creating stable structures we call molecules. Think of molecules as tiny dance partners, with each atom swaying to the rhythm of shared electrons.
Like all relationships, chemical bonds come in different types. The most common is the covalent bond, where atoms share electrons to create an invisible web of attraction. In covalent molecules, like water (H2O), electrons are like the shared thread that binds hydrogen and oxygen atoms together.
The Difference Between Compounds and Elements
While compounds and elements share a common ancestor – atoms – they are distinct entities. Elements are pure substances made up of only one type of atom. Think of gold, a shiny metal composed solely of gold atoms. In contrast, compounds are formed when different atoms chemically bond to form new substances with unique properties. For instance, table salt (NaCl) is a compound composed of sodium (Na) and chlorine (Cl) atoms that have joined forces to form a crystalline structure.
The Significance of Chemical Bonding
Chemical bonding is the secret sauce that gives compounds their remarkable properties. It determines not only the structure of molecules but also their reactivity, color, and countless other characteristics. For example, the covalent bonds in sugar give it its sweet taste, while the ionic bonds in salt make it soluble in water.
Compounds, the result of atoms’ harmonious collaboration, are the building blocks of the world around us. From the oxygen we breathe to the plastic in our computers, compounds play a crucial role in our lives. Understanding the basic principles of chemical bonding is essential for unraveling the mysteries of matter and appreciating the intricate tapestry of our physical world.
Subatomic Particles: The Building Blocks of Atoms
At the heart of every atom lies a universe of tiny particles, each playing a pivotal role in shaping the matter that surrounds us. These subatomic particles – protons, neutrons, and electrons – are the fundamental building blocks of atoms, the basic units of all matter.
Protons, the positively charged particles, reside in the nucleus of the atom, the dense center that contains most of the atom’s mass. Their number determines the element to which an atom belongs. For instance, all atoms with one proton are hydrogen atoms, while those with six protons are carbon atoms.
Neutrons, on the other hand, are uncharged particles that also occupy the nucleus alongside protons. They contribute to an atom’s mass, but do not influence its chemical identity. Neutrons act as a balancing force, stabilizing the nucleus and preventing protons from repelling each other.
Electrons, in contrast to protons and neutrons, are negatively charged particles that orbit the nucleus in designated energy levels. These energy levels determine the electron’s distance from the nucleus and its involvement in chemical bonding. Electrons are responsible for an atom’s chemical reactivity, as they interact with electrons from other atoms to form molecules and compounds.
Atomic Properties: Number and Mass
Every element on the periodic table has a unique identity defined by its atomic properties, including its atomic number and mass number. Understanding these properties is crucial for comprehending the behavior and interactions of elements in the world around us.
The atomic number of an element represents the number of protons in its nucleus. Protons carry a positive charge, and their number determines the element’s position on the periodic table. For example, all atoms with one proton are hydrogen atoms, while those with two protons are helium atoms.
The mass number of an element, on the other hand, represents the total number of protons and neutrons in its nucleus. Neutrons, unlike protons, have no charge and contribute to the atom’s mass. The difference between the mass number and the atomic number gives the number of neutrons in an atom.
These two properties together play a vital role in determining an element’s identity and its behavior in chemical reactions. Elements with the same atomic number but different mass numbers are known as isotopes. Isotopes have the same chemical properties but differ in their neutron count and therefore their mass.
For example, carbon-12 (C-12) has six protons and six neutrons, while carbon-14 (C-14) has six protons but eight neutrons. Both isotopes have the same chemical properties, but C-14 is radioactive and decays over time.
Isotopes are important for a variety of applications, including dating fossils and diagnosing medical conditions. They also play a role in understanding nuclear reactions and the development of new technologies.
Another related concept is isobars, which are atoms of different elements that have the same mass number but different atomic numbers. Isobars have different chemical properties but share a similar atomic mass. For example, calcium-40 (Ca-40) and potassium-40 (K-40) are both isobars with a mass number of 40 but different atomic numbers (20 and 19, respectively).
By understanding atomic number and mass number, we gain insights into the fundamental properties of elements and their behavior in the world around us. These concepts are essential for students of chemistry, physics, and other scientific disciplines, as well as for anyone interested in the inner workings of matter.
Ions: The Electrically Charged Atoms
Imagine a world where atoms aren’t just neutral entities but can carry electrical charges. Meet ions, the electrically charged counterparts of atoms. These charged particles play crucial roles in various chemical reactions and biological processes.
Formation of Ions
Atoms can transform into ions by either losing or gaining electrons. When an atom loses electrons, it becomes a positively charged ion called a cation. Conversely, when an atom gains electrons, it forms a negatively charged ion known as an anion.
For instance, sodium (Na) atom loses one electron, resulting in a sodium cation (Na+). Chlorine (Cl), on the other hand, gains one electron to become a chloride anion (Cl-).
Properties and Significance of Ions
Ions possess unique properties that distinguish them from neutral atoms. These charged particles are highly reactive and readily engage in chemical reactions. Their electrical charges allow them to attract oppositely charged ions, forming ionic bonds.
In living organisms, ions play vital roles in maintaining electrical gradients across cell membranes. They also regulate nerve impulses and muscle contractions.
Examples of Ion Formation and their Significance
- Sodium-potassium pump: This ion pump in cell membranes actively transports sodium ions (Na+) out of cells and potassium ions (K+) into cells. This process helps maintain electrical gradients and cellular homeostasis.
- Acid-base reactions: Ions are fundamental in acid-base reactions. Acids, like hydrochloric acid (HCl), dissociate in water to release positively charged hydrogen ions (H+). Bases, like sodium hydroxide (NaOH), release negatively charged hydroxide ions (OH-). The interaction between H+ and OH- ions leads to neutralization reactions and determines the pH of solutions.
- Electrochemical cells: Ions are key components in electrochemical cells, such as batteries and fuel cells. These devices convert chemical energy into electrical energy through the movement of ions across a semipermeable membrane.
Ions, the electrically charged forms of atoms, are essential building blocks of matter. Their unique properties and reactivity make them key players in a wide range of chemical and biological processes. Understanding the formation and properties of ions provides a deeper appreciation of the intricate world of chemistry and its implications in our daily lives.
The Periodic Table: A Blueprint for Understanding Elements
The periodic table is a masterpiece of scientific organization, a comprehensive guide to the building blocks of our universe. It’s a visual symphony that reveals the fundamental patterns and relationships among the elements, the basic units of matter.
The periodic table is structured like a table, with rows (periods) and columns (groups). Each element occupies a specific position based on its atomic number, which is the number of protons in its nucleus. The atomic number also defines an element’s identity.
The arrangement of elements in the periodic table is not arbitrary. It reflects their electron configurations, the arrangement of electrons around the atomic nucleus. Elements in the same group have similar electron configurations, which in turn determines their chemical properties.
For example, the first group contains elements with one valence electron (an electron in the outermost shell). These elements, like sodium and potassium, are highly reactive and tend to lose their valence electron easily. In contrast, elements in the last group, known as noble gases, have full valence shells and are extremely unreactive.
The periodic table is a powerful tool for predicting element properties. By knowing an element’s position in the table, scientists can infer its chemical reactivity, physical properties, and even its biological significance. For example, elements in the same period tend to have similar sizes and ionization energies.
The periodic table is a testament to the interconnectedness of nature. It reveals how the smallest components of matter, the elements, combine to create the vast diversity of substances that make up our world. From the air we breathe to the devices we use, the periodic table is a blueprint for understanding the fundamental building blocks of our universe.