Temperature’s Influence On Ph: Impact Of Ionization, Pka, And Buffers
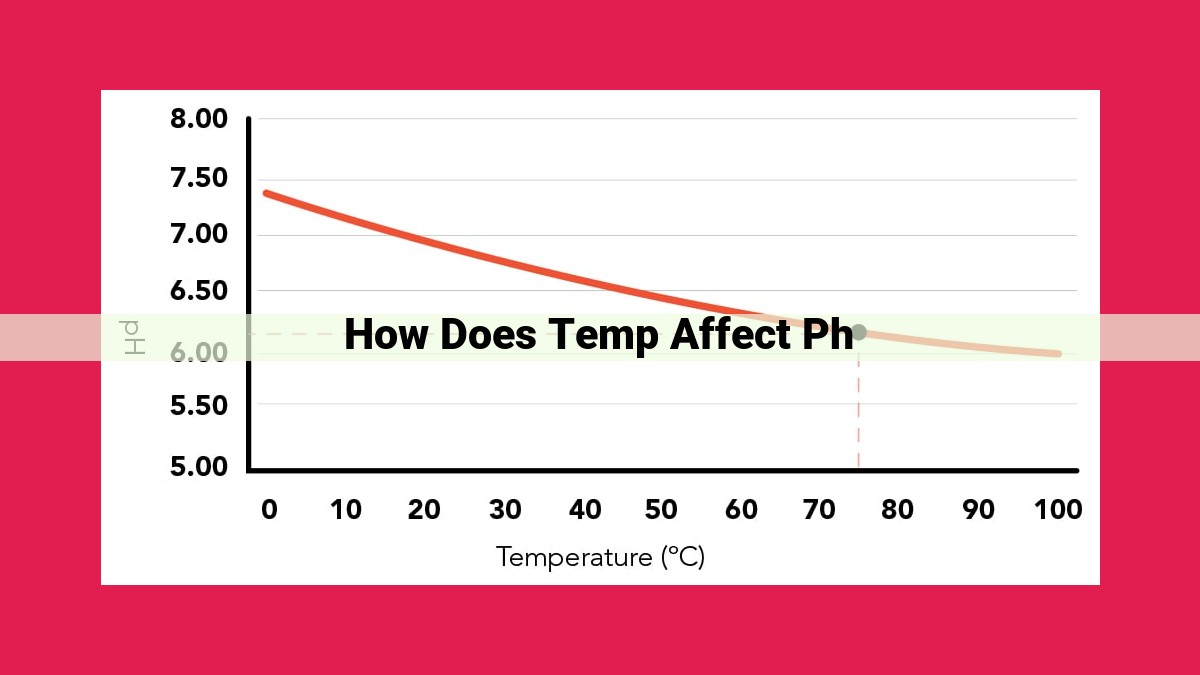
Temperature affects pH due to several factors, including the temperature coefficient of pH, dissociation constant (pKa), and ionization of water. As temperature increases, the pH of solutions generally decreases. This is because higher temperatures increase the ionization of acids and bases, leading to the release of more H+ ions and the reduction of pH. The effect of temperature on pH is influenced by the strength of the acid or base, ionic strength, activity coefficients, and the presence of buffer systems.
Temperature Coefficient of pH:
- Definition and relationship to pH and temperature
- Explanation of how pH decreases with increasing temperature due to increased ionization and production of H+ ions
The Puzzling Dance of pH and Temperature: Unraveling the Secrets
If you’ve ever wondered why the pH of your swimming pool changes with the seasons, or why your favorite acidic juice tastes different on a hot day, you’ve stumbled upon the fascinating interplay between pH and temperature. Let’s dive into the scientific tale behind this curious phenomenon.
The Temperature Coefficient of pH: A Tale of Ions
Picture a pH scale, a spectrum ranging from acidic to basic. Now, imagine the scale getting shorter as the temperature rises. That’s because temperature has a profound effect on the concentration of hydrogen ions (H+), which are responsible for acidity.
As the temperature goes up, H+ ions become more energetic and break free from their chemical bonds, leading to increased ionization. This surge in H+ ions pushes the pH scale towards the acidic end. Hence, the pH of a solution decreases with increasing temperature.
Understanding Ionization and Dissociation
Ionization is the key to comprehending the temperature coefficient of pH. When molecules or ions dissolve in water, they can break apart, releasing ions. The dissociation constant (pKa) is a measure of how strongly an acid dissociates in water.
Temperature influences pKa. As temperature rises, pKa values tend to decrease, meaning acids become stronger and bases become weaker. This shift further contributes to the decrease in pH with increasing temperature.
The Role of Water Ionization
Water itself undergoes ionization, forming H+ and hydroxide (OH-) ions. This ionization constant (Kw) also increases with temperature, leading to a higher concentration of H+ ions and an overall decrease in pH.
Additional Factors to Consider
While temperature is a major factor, other aspects can impact pH. Ionic strength, the concentration of dissolved ions, and activity coefficients, which account for non-ideal ion behavior, can influence pH to varying degrees.
The Practical Implications
Understanding the temperature coefficient of pH is crucial in diverse fields. In biology, it affects enzyme activity and metabolic processes. In environmental chemistry, it influences the behavior of aquatic organisms and the fate of pollutants. And in industry, it affects the stability and performance of products.
The dance between pH and temperature is a testament to the intricate relationships within our natural world. By unraveling these secrets, we gain a deeper appreciation for the dynamic nature of chemical systems and the impact of temperature on everyday phenomena.
The Dissociation Constant: Understanding Its Dependence on Temperature
In the realm of chemistry, the dissociation constant, better known as pKa, plays a pivotal role in determining the strength of acids and bases. It measures the tendency of an acid to lose a proton (H+ ion) and form its conjugate base. But did you know that temperature can significantly influence the pKa of a compound?
The pKa value is inversely proportional to the strength of an acid. The lower the pKa, the stronger the acid, and the more readily it donates protons. Conversely, the higher the pKa, the weaker the acid, and the less likely it is to release protons.
Temperature affects the pKa of an acid because it influences the degree of ionization. As temperature increases, the ionization of the acid also increases. This means that more protons are released, leading to a decrease in the pKa. The acid becomes stronger with increasing temperature.
On the other hand, a decrease in temperature reduces ionization, resulting in fewer protons being released. This causes the pKa to increase, making the acid weaker.
This temperature dependence of pKa has significant implications in various chemical processes. For example, in biological systems, where temperature fluctuations are common, it can affect the activity of enzymes and the pH balance of cells. In industrial settings, temperature control is crucial for optimizing chemical reactions that rely on acid-base equilibria.
Understanding the relationship between pKa and temperature is essential for manipulating acid-base behavior in different contexts. By adjusting temperature, we can control the extent of ionization and tune the strength of acids and bases, facilitating precise control of chemical reactions and processes.
Ionization of Water: Temperature’s Influence on pH
In the realm of chemistry, the ionization of water holds an important place. Water, a seemingly innocuous substance, harbors a hidden power that unfolds when exposed to temperature variations. Let’s plunge into the captivating story of how temperature wields its influence over water’s ionization and unveils the dynamic nature of pH.
At its core, the process of ionization entails the splitting of water molecules (H2O) into their ionic constituents: hydrogen ions (H+) and hydroxide ions (OH-). This phenomenon occurs naturally, albeit to a limited extent, endowing water with its amphoteric character – the ability to act as both an acid and a base.
Crucially, the ionization constant of water (Kw), a numerical value that quantifies the extent of ionization, is not static but rather temperature-dependent. As temperature rises, Kw increases, reflecting a greater propensity for water to dissociate into its ions.
This temperature-driven shift in Kw has a profound impact on the pH, a measure of a solution’s acidity or basicity. pH scales from 0 to 14, with values below 7 indicating acidity, above 7 indicating basicity, and exactly 7 denoting neutrality.
Intriguingly, the inverse relationship between temperature and pH stems from this temperature dependency of Kw. As temperature increases, Kw increases, leading to greater ionization of water. This surge in H+ ions pushes the pH downward, rendering solutions more acidic.
In essence, the ionization of water is a temperature-sensitive process, shaping the pH of aqueous solutions. Understanding this relationship is paramount in various scientific and industrial applications, ranging from chemical reactions to environmental monitoring, where temperature’s influence on pH plays a pivotal role in determining the behavior and stability of systems.
pH: A Measure of Acidity and Basicity
What is pH?
pH is a measure of how acidic or basic a solution is. It is expressed on a scale from 0 to 14, with 0 being the most acidic and 14 being the most basic. A pH of 7 is neutral.
The Temperature Dependence of pH
pH is temperature-dependent. As temperature increases, the pH of a solution decreases. This is because higher temperatures increase the ionization of water, which produces more hydrogen ions (H+). Hydrogen ions are responsible for acidity, so the more hydrogen ions there are, the more acidic the solution becomes. As a result, the pH decreases with increasing temperature.
Implications of Temperature on pH
The temperature dependence of pH has important implications for many areas of chemistry and biology, including:
- Chemical reactions: Temperature affects the equilibrium constants of chemical reactions that involve acids or bases. This can alter the rates and outcomes of these reactions.
- Biological processes: pH is crucial for the proper functioning of many biological systems. For example, the pH of blood is tightly regulated, and changes in blood pH can have serious consequences for health.
- Environmental monitoring: pH is used to monitor the health of ecosystems. Changes in pH can indicate pollution or other environmental disturbances.
Understanding the temperature dependence of pH is essential for a wide range of scientific and practical applications.
How Ionic Strength Influences pH: A Deeper Dive
In the world of chemistry, understanding the intricacies of pH is crucial. While various factors can affect pH, one often overlooked element is ionic strength. Let’s unravel the fascinating interplay between ionic strength and pH.
Ionic strength, a measure of the concentration of ions in a solution, plays a significant role in determining its acidity or alkalinity. Higher ionic strength solutions contain more ions, which can compete with the hydrogen ions (H+) for water molecules. This competition leads to a decrease in the activity of H+ ions and, subsequently, a decrease in pH.
In other words, as ionic strength increases, the solution becomes less acidic and may even become basic. This phenomenon is particularly evident in solutions of strong acids and bases, where the high concentration of ions suppresses the dissociation of the acid or base, resulting in a lower concentration of H+ or hydroxide ions (OH-).
The impact of ionic strength on pH is not to be underestimated. It is a critical consideration in various fields, including environmental chemistry, biochemistry, and industrial processes. By comprehending the relationship between ionic strength and pH, scientists and engineers can optimize reactions, control chemical processes, and better understand the behavior of aqueous solutions in various contexts.
Activity Coefficients and Their Impact on pH
Activity coefficients are crucial factors that account for the deviation from ideal behavior exhibited by ions in solutions. These coefficients are essentially correction factors that adjust the concentrations of ions to account for their non-ideal interactions with each other and with the solvent molecules.
As temperature changes, so do the activity coefficients of ions. This is because temperature influences the solvation of ions, which refers to the interaction between ions and the surrounding solvent molecules. Changes in solvation can affect the effective concentration of ions in solution, ultimately impacting pH.
For instance, in aqueous solutions, increasing temperature generally leads to decreased activity coefficients for ions. This is because the solvent molecules become more energetic and break apart the ion-solvent interactions, resulting in a more “free” state for the ions. Consequently, the effective concentration of ions increases, which can lead to a decrease in pH.
Conversely, decreasing temperature typically results in increased activity coefficients for ions. In this case, the solvent molecules become less energetic and strengthen their interactions with ions, effectively reducing the concentration of “free” ions. This decrease in effective ion concentration can lead to an increase in pH.
Understanding the influence of activity coefficients on pH is essential in various fields, such as chemistry, environmental science, and biochemistry. It allows researchers and practitioners to accurately predict and control the acid-base behavior of solutions under varying temperature conditions. By considering activity coefficients, it becomes possible to ensure optimal conditions for chemical reactions, maintain pH stability in biological systems, and enhance the effectiveness of pH-dependent processes.
Hydrolysis: When Salts React with Water
In the world of chemistry, water isn’t just a neutral backdrop; it’s an active participant in many reactions. One of these reactions is hydrolysis, where salts, seemingly inert compounds, dance with water to create acids or bases.
Imagine a salt like sodium acetate. When it meets water, the sodium ions and acetate ions separate. However, water has a secret power: it can act as both an acid and a base. In this case, water’s basic side attracts the hydrogen ions from the acetate ions, creating acetic acid.
The extent to which a salt hydrolyzes depends on several factors, including the strength of the acid and base it forms and the temperature. As temperature rises, the equilibrium constant for hydrolysis also increases, meaning more salt molecules break down.
This temperature dependence is crucial because it affects the pH of solutions. As hydrolysis increases, the pH decreases, making the solution more acidic. For example, a solution of sodium acetate at room temperature has a pH of around 8.2, but at higher temperatures, it becomes more acidic.
Understanding hydrolysis is essential in various fields, such as medicine and biotechnology. By controlling the temperature and salt concentration, scientists can fine-tune the pH of solutions to create specific environments for reactions or to study the behavior of biological systems.
Buffer Capacity and Temperature: A Tale of pH Stability
In the realm of chemistry, pH plays a crucial role in determining the acidity or basicity of a solution. While pH is often measured at room temperature, it’s essential to understand how temperature affects this vital parameter. One factor that significantly influences pH is buffer capacity.
Buffer solutions are like the gatekeepers of pH, preventing drastic changes when acids or bases are added. They contain a weak acid and its conjugate base (or vice versa) and resist pH shifts within a specific range.
The Temperature Dance
Temperature plays a delicate game with buffer capacity. As temperature rises, the ionization of both the weak acid and its conjugate base increases. This increase in ionization leads to a decrease in the buffer capacity of the solution. The reason behind this is that the buffer is now more susceptible to pH changes upon the addition of acids or bases.
The Buffer Components
The strength of the acid and base components within the buffer also influences its temperature sensitivity. A buffer composed of a strong acid and its weak conjugate base will be less affected by temperature changes than one containing a weak acid and its strong conjugate base.
Real-World Implications
Understanding the temperature dependence of buffer capacity is crucial in various chemical and biological applications. For instance, in biochemical systems, maintaining a stable pH is vital for enzyme activity and cellular processes. Buffers with appropriate temperature-insensitive properties are essential for maintaining optimal pH conditions.
In pharmaceutical formulations, buffers are used to ensure the stability and efficacy of drugs over a range of temperatures, especially during storage and transport. The selection of buffer components and their temperature dependence is critical to preserve the drug’s desired pH profile.
Buffer capacity is a dynamic property that responds to changes in temperature. By understanding how temperature affects the ionization of buffer components, we can optimize their use in various applications. From maintaining pH stability in biological systems to ensuring the integrity of pharmaceuticals, the temperature dependence of buffer capacity is a key consideration in the world of chemical and biological sciences.