Understanding The Effect Of Temperature On Ph: A Comprehensive Guide
Temperature influences pH by altering the dissociation of water molecules. As temperature increases, the water dissociation constant (Kw) decreases, leading to fewer H+ and OH- ions in solution and a shift in equilibrium towards undissociated water. Consequently, the H+ ion concentration decreases, causing an increase in pH and making the solution more basic. This temperature dependency is described by the van’t Hoff Equation, which quantifies the relationship between temperature and equilibrium constants.
Understanding pH and Temperature
- Define pH as a measure of acidity or alkalinity.
- Explain temperature’s impact on the dissociation of water molecules.
Understanding pH and Temperature: A Deeper Dive
pH, a measure of acidity or alkalinity, plays a crucial role in various chemical and biological processes. It is determined by the concentration of hydrogen ions (H+) in a solution. Temperature, on the other hand, has a significant impact on the dissociation of water molecules and, consequently, the pH of a solution.
Impact of Temperature on Water Dissociation
Water molecules tend to dissociate into hydrogen ions (H+) and hydroxide ions (OH-). This dissociation is affected by temperature. As temperature increases, the kinetic energy of water molecules increases, leading to a greater number of collisions between them. This increased collision frequency favors the breaking of bonds between H+ and OH- ions, resulting in more dissociation and a higher concentration of H+.
Equilibrium Shift and Le Chatelier’s Principle
According to Le Chatelier’s Principle, if a change is made to an equilibrium system, the system will shift in a direction that opposes the change. In the case of water dissociation, an increase in temperature favors the dissociation reaction (formation of H+ and OH- ions). Therefore, to counteract this shift, the equilibrium moves toward reassociation (combination of H+ and OH- ions to form water molecules). This equilibrium shift results in a lower concentration of H+ ions and, consequently, a higher pH (less acidic).
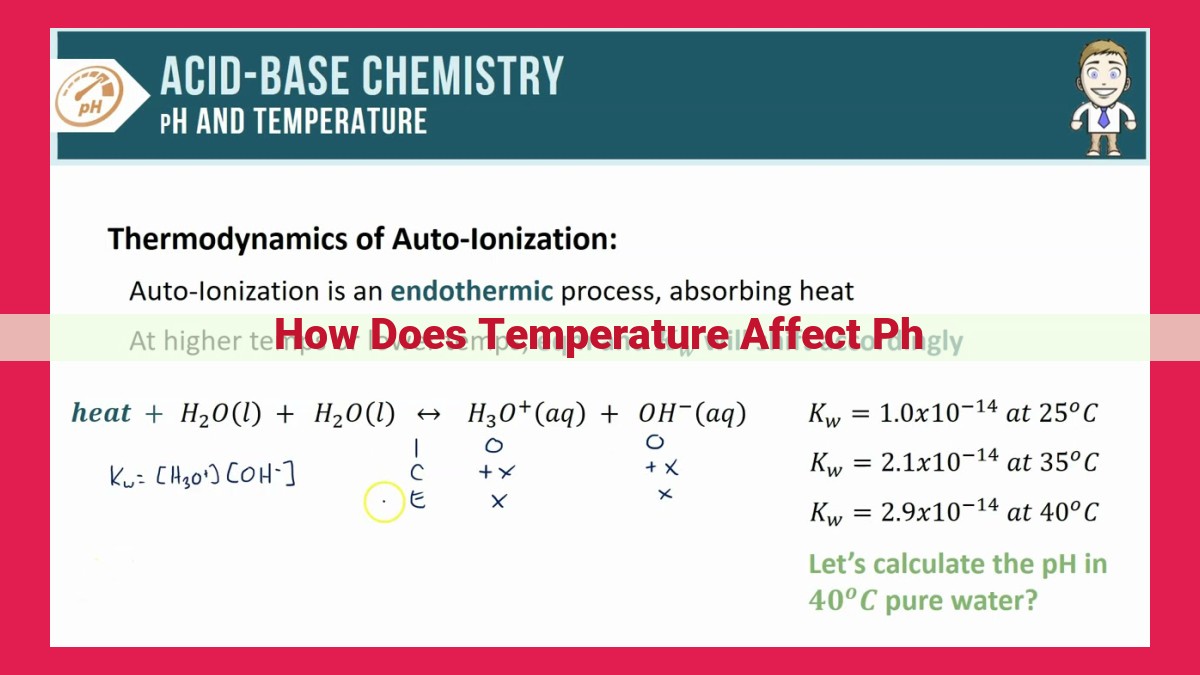
Temperature Dependency of Water’s Dissociation Constant
Water, the elixir of life, is not just a simple molecule but a fascinating chemical system. At the heart of this system lies the concept of pH, a measure of the acidity or alkalinity of a solution. Temperature, an equally important factor, plays a crucial role in shaping the pH of water.
Water’s Ions
In its pure form, water undergoes a process known as autoionization or autoprotolysis, where it splits into hydrogen ions (H+) and hydroxide ions (OH-). The extent of this dissociation is governed by the dissociation equilibrium constant (Kw).
Temperature’s Impact on Kw
Crucially, temperature exerts a profound influence on the value of Kw. As temperature increases, the dissociation of water increases as well. This means that more water molecules break down into H+ and OH- ions, resulting in a higher Kw value. Conversely, as temperature decreases, the dissociation of water decreases, and Kw decreases accordingly.
Equilibrium Shift
The temperature dependence of Kw can be understood using Le Chatelier’s Principle. According to this principle, if a change is made to an equilibrium system, the system will shift to counteract the change. In this case, increasing temperature shifts the equilibrium away from dissociation because the addition of heat favors the recombination of H+ and OH- ions. This results in a lower Kw value.
Implications
The temperature dependency of Kw has profound implications for various scientific disciplines, including environmental studies and biological systems. In environmental science, understanding the temperature sensitivity of water’s pH is crucial for predicting the impact of climate change on aquatic ecosystems. In biology, the temperature dependence of Kw affects the pH of biological fluids, which in turn influences the function and behavior of enzymes and proteins.
Temperature Coefficients
Scientists have developed temperature coefficients to quantify the changes in Kw with temperature. The van’t Hoff Equation is a mathematical expression that describes this relationship, allowing researchers to predict the value of Kw at different temperatures.
Equilibrium Shift and Le Chatelier’s Principle
When it comes to the equilibrium of water dissociation, the temperature has a say. Picture this: In the world of chemistry, we have a concept called Le Chatelier’s Principle, which states that if you apply stress to a system at equilibrium, the system will shift to counteract that stress and re-establish equilibrium.
Now, let’s apply this principle to water dissociation. When temperature increases (the stress), we observe that the dissociation of water shifts away from dissociation. This means that the equilibrium shifts to favor the formation of undissociated water molecules (H2O).
This shift can be explained by the fact that increasing temperature raises the kinetic energy of water molecules. As a result, the molecules move more vigorously and experience more collisions with each other. This increased collision frequency leads to a higher probability of recombination, resulting in the formation of more H2O molecules and a decrease in the dissociation equilibrium constant (Kw).
So, in summary, as temperature rises, the equilibrium shifts away from dissociation, leading to a lower Kw and, ultimately, a higher pH (more basic). This phenomenon is crucial in understanding various chemical and biological processes that are temperature-dependent.
Ionization of Water: The Dance of Hydrogen and Hydroxide Ions
Every drop of water, as humble as it may seem, conceals a captivating tale of molecular drama. Within this aqueous realm, an intricate dance unfolds between hydrogen (H+) and hydroxide (OH-) ions – a dance known as autoprotolysis.
In this enchanting dance, water molecules spontaneously split into their constituent ions: H+ and OH-. This process, like a delicate chemical ballet, gives water its unique capacity to dissolve a myriad of substances and play a crucial role in countless chemical reactions.
The key to understanding autoprotolysis lies in the remarkable equality of hydrogen and hydroxide ion concentrations in pure water. At room temperature, the [H+] and [OH-] concentrations are exquisitely balanced, each hovering around 1 x 10^-7 moles per liter.
This delicate equilibrium, like a graceful waltz, is maintained by the dynamic nature of water molecules. As some water molecules dissociate, forming H+ and OH- ions, others recombine to form undissociated H2O molecules. This constant exchange ensures a harmonious balance between the two ions.
Temperature’s Effect on pH: A Tale of Shifting Equilibria
Imagine a bustling metropolis where tiny molecules, H+ and OH-, dance around in a constant exchange of partners. This intricate ballet is known as the ionization of water, a delicate process that determines the acidity or alkalinity—the pH—of our aqueous world.
An Equilibrium Dance
The ionization of water is a delicate equilibrium, a perfect balance between the formation and dissociation of H+ and OH- ions. At room temperature, about one out of every 107 water molecules dissociates, giving rise to equal concentrations of H+ and OH- ions. This blissful harmony results in a neutral pH of 7.
The Temperature Factor
But like a graceful dance disrupted by the arrival of an unexpected guest, the temperature can upset the delicate equilibrium of water dissociation. As temperature rises, the number of dissociating water molecules dwindles. This shift is akin to a ballroom becoming less crowded, with fewer ions twirling in the space.
Consequences for Acidity
The diminishing number of H+ ions has a profound impact on the pH: it increases. This means that the solution becomes less acidic or more basic. In essence, as temperature rises, the once-neutral water becomes more “friendly” to OH- ions, shifting the balance of power in their favor.
The temperature’s influence on pH is a fascinating chemical dance, a testament to the intricate interconnectedness of our molecular world. Understanding this interplay is crucial for a wide range of disciplines, from chemistry and biology to environmental science and industrial processes. So, the next time you raise the temperature of a glass of water, remember the hidden drama unfolding within, where the equilibrium ballet of ions and pH takes center stage.
Temperature Coefficients and van’t Hoff Equation
In the captivating dance of chemical reactions, temperature plays a pivotal role. It can sway the delicate balance of equilibrium, shifting the landscapes of our chemical environments. To unravel the mysteries of this temperature-induced dance, scientists have devised ingenious tools like temperature coefficients and the van’t Hoff Equation.
Temperature Coefficients: Measuring the Dance of Equilibrium
Imagine a teetering seesaw, precariously balancing two opposing forces. Temperature coefficients, symbolized by the Greek letter “gamma”, capture the extent to which equilibrium constants respond to the relentless beat of temperature. Just as the seesaw tilts with the slightest nudge, a temperature coefficient quantifies the magnitude and direction of this dance.
van’t Hoff Equation: Unraveling the Enigma of Temperature Dependence
To unravel the secrets of how temperature entwines with equilibrium constants, we turn to the enigmatic van’t Hoff Equation. This mathematical marvel relates the temperature coefficient, the change in enthalpy associated with the reaction, and the all-important temperature. It paints a vivid picture of the temperature’s grip on the interplay of chemical species.
By harnessing the power of temperature coefficients and the van’t Hoff Equation, scientists can decipher the choreography of equilibrium in a myriad of chemical reactions. From the subtle shifts in water’s dissociation to the fiery exchanges in combustion, these tools illuminate the intricate relationship between temperature and the delicate tapestry of chemical transformations.