Tellurium: A Unique Metalloid With Versatile Applications In Advanced Technologies

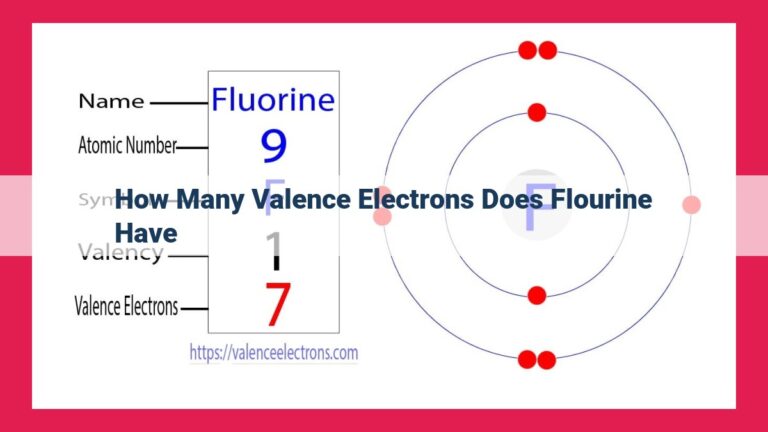
Tellurium, a metalloid with unique electronic properties, has four valence electrons. Its electron configuration reveals valence electrons in the 6p subshell, which are responsible for its chemical behavior. The number of valence electrons determines a substance’s reactivity, electronegativity, and ability to form chemical bonds, impacting tellurium’s applications in various fields, such as semiconductors, solar cells, and medicinal compounds.
Understanding Valence Electrons: The Cornerstone of Chemical Properties
In the captivating realm of chemistry, valence electrons play a pivotal role in unraveling the mysteries of chemical reactions and the behavior of elements. These elusive electrons, found in the outermost energy level of an atom, hold the key to understanding the element’s properties and reactivity.
Definition of Valence Electrons
Valence electrons are the electrons that occupy the highest energy level, or valence shell, of an atom. They are the most loosely bound electrons, making them highly reactive and eager to participate in chemical bonding.
Significance of Valence Electrons in Chemical Properties
The number and arrangement of valence electrons dictate an element’s chemical properties. For instance, elements with a full valence shell are typically stable and inert, while those with incomplete valence shells are more reactive and eager to interact with other atoms.
Quantum Numbers and Atomic Orbitals
The behavior of valence electrons can be further explained through the concept of quantum numbers and atomic orbitals. Quantum numbers describe the energy, shape, and orientation of atomic orbitals, which are the spatial regions where electrons are most likely to be found. By understanding these principles, scientists can predict the behavior and properties of elements based on their valence electron configuration.
Tellurium: A Metalloid with a Unique Blend
Nestled in the periodic table, far from the bustling metals and secluded from the nonmetals, lies the enigmatic element known as tellurium. This extraordinary substance defies easy categorization, occupying the ambivalent realm of metalloids.
Tellurium’s metallic prowess manifests in its shimmering silver-white luster and its remarkable electrical conductivity, traits that hint at its affinity with its metallic brethren. Yet, like a cunning chameleon, tellurium also possesses nonmetallic qualities: it’s brittle, susceptible to hydrolysis, and forms oxides that exhibit acidic properties. This intriguing duality makes tellurium a scientific enigma, an element that dances between two worlds.
In the periodic table, tellurium resides in the halogens column, nestled beneath selenium and above polonium. Its atomic number of 52 signifies its possession of 52 electrons, of which four are its valence electrons. These valence electrons hold the key to understanding tellurium’s unique properties and extraordinary versatility in the world of chemistry.
Tellurium’s Electron Configuration: Unveiling the Secrets of a Metalloid
In the realm of chemistry, the electron configuration of elements holds the key to unraveling their unique properties and behavior. Tellurium, a fascinating metalloid, captivates scientists with its enigmatic electron arrangement.
Tellurium’s atomic number, 52, indicates its 52 electrons. By applying the principles of quantum mechanics, we can unveil the distribution of these electrons:
- Core Electrons: The innermost electrons, tightly bound to the nucleus, form the core.
- Valence Electrons: The outermost electrons, occupying the highest energy level, are crucial for chemical bonding and determine an element’s reactivity.
Tellurium’s electron configuration can be simplified as 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁶ 6s² 6p⁴. This notation reveals that tellurium has four valence electrons in its 6p subshell.
The presence of four valence electrons bestows tellurium with peculiar properties:
- Reactivity: Tellurium’s valence electrons make it prone to forming chemical bonds, contributing to its versatile bonding behavior.
- Metalloid Characteristics: As a metalloid, tellurium exhibits properties of both metals and nonmetals. Its four valence electrons allow it to form both metallic and nonmetallic bonds.
Understanding tellurium’s electron configuration is paramount for comprehending its chemical properties and its applications in various fields. From semiconductors to solar cells and medicinal compounds, tellurium’s unique electron configuration unlocks its potential for technological advancements and scientific breakthroughs.
Electronic Properties and Reactivity of Tellurium
Tellurium, an enigmatic metalloid nestled in the periodic table, possesses unique electronic properties that determine its chemical bonding behavior and reactivity. Let’s unravel the intricacies of tellurium’s electronic landscape and witness the fascinating dance it embarks upon with other elements.
Tellurium’s electronegativity, a measure of its ability to attract electrons towards itself, is moderate. This intriguing property grants tellurium the ability to form both covalent and ionic compounds, allowing it to adapt seamlessly to a diverse array of chemical environments.
When tellurium encounters elements with higher electronegativity, such as oxygen and halogens, it tends to donate its valence electrons. This electron-giving prowess results in the formation of ionic compounds with tellurium assuming a positive charge.
In stark contrast, tellurium displays a remarkable ability to share its valence electrons when interacting with elements of lower electronegativity, such as metals. These electron-sharing escapades give rise to covalent compounds where tellurium proudly flaunts its coordination ability, forming bonds with multiple atoms simultaneously.
Tellurium’s versatile electronic nature extends beyond its bonding preferences. Its reactivity with other elements is a testament to its eagerness to engage in chemical transformations. Tellurium eagerly reacts with oxygen to form tellurium dioxide, a compound commonly employed in the production of glass and ceramics.
Delving deeper into tellurium’s chemical adventures, we discover its affinity for halogens. Tellurium readily combines with chlorine, bromine, and iodine to yield tellurium halides, which serve as valuable intermediates in various chemical reactions.
Unveiling the electronic properties and reactivity of tellurium unveils a fascinating world of chemical possibilities. Tellurium’s ability to chameleon-like adapt its bonding behavior and reactivity based on its environment makes it an indispensable player in a myriad of chemical applications, from semiconductors to solar cells, and even medicinal compounds.
Tellurium: A Versatile Metalloid with Diverse Applications
In the realm of chemistry, tellurium stands out as a unique metalloid, boasting a remarkable array of properties that render it indispensable in various technological and medicinal applications.
Semiconductors and Solar Cells
Tellurium’s electronic properties play a pivotal role in its use as a semiconductor material. Semiconductors are essential components in transistors, microchips, and other electronic devices. Tellurium’s four valence electrons enable it to form p-type semiconductors, which are crucial for the flow of electricity in these devices.
Moreover, tellurium’s ability to absorb infrared radiation makes it an ideal material for solar cells. When combined with other elements such as cadmium, tellurium forms cadmium telluride (CdTe), a highly efficient semiconductor used in the production of solar panels.
Medicinal Compounds
Tellurium’s electronic properties also contribute to its pharmacological applications. Organic tellurium compounds have been investigated as potential antimicrobial and antitumor agents. Their ability to inhibit the growth of cancer cells and bacteria has garnered significant interest in the medical community.
In particular, tellurium-containing compounds have shown promise in combating antibiotic-resistant bacteria, a growing concern in modern healthcare.
Other Applications
Beyond semiconductors and medicinal compounds, tellurium finds applications in various other fields:
- Alloys: Tellurium is added to steel to improve its machinability and corrosion resistance.
- Catalysis: Tellurium compounds are used as catalysts in chemical reactions, facilitating the production of plastics, pharmaceuticals, and other materials.
- Photography: Tellurium is used in the production of photographic film.
- Thermoelectrics: Tellurium is employed in thermoelectric materials that convert heat into electricity.
Tellurium, with its unique electronic properties and versatility, is a remarkable metalloid with a wide range of applications in modern technology and medicine. From semiconductors to solar cells, and from antimicrobial agents to photographic film, tellurium’s contributions are truly diverse. As research continues to unravel the potential of this enigmatic element, we can anticipate even more groundbreaking applications in the future.