Understanding Sulfuric Acid Neutralization: A Comprehensive Guide

Neutralizing sulfuric acid involves using bases to reduce its acidity. Antacids, containing bases, can neutralize stomach acid, relieving heartburn. Weak acids can also neutralize sulfuric acid, forming water and salt. By performing a neutralization reaction, using a base to neutralize the acid, and identifying the equivalence point using a pH indicator, the exact amount of base required for neutralization can be determined through titration.
Neutralization: The Power of Bases in Taming Acids
What is Neutralization?
When an acid and a base come together, they embark on a chemical dance known as neutralization. This reaction transforms the acidic and corrosive nature of acids into something more manageable.
The Role of Bases in Neutralization
Bases, like antacids and alkalis, possess the ability to neutralize acids. They do this by providing hydroxide ions (OH-), which have a negative charge. These ions interact with the positively charged hydrogen ions (H+) in acids, forming water (H2O) as a byproduct. This process effectively reduces the acidity and corrosive properties of the acid.
Applications of Neutralization
-
Antacids for Stomach Relief: Antacids are a common remedy for heartburn and indigestion. They contain bases that neutralize stomach acid, providing relief from discomfort.
-
Neutralizing Sulfuric Acid: Weak acids, such as acetic acid, can be used to neutralize sulfuric acid. When these acids react, they form water and salts, such as sodium sulfate. This reaction is commonly used in industrial processes.
Performing a Neutralization Reaction
A neutralization reaction involves the following steps:
- Combine an acid and a base in the right proportions.
- The hydrogen ions (H+) in the acid react with the hydroxide ions (OH-) in the base.
- This reaction forms water (H2O) and a compound known as a salt.
Identifying the Equivalence Point
The equivalence point marks the moment when the acid and base have been completely neutralized. This point can be identified using a pH indicator, a substance that changes color depending on the acidity or alkalinity of the solution. When the equivalence point is reached, the pH indicator will indicate a neutral pH.
Titration for Precise Neutralization
Titration is a technique used to determine the exact amount of base required to neutralize an acid. By gradually adding a base to an acid solution while monitoring the pH, the equivalence point can be precisely determined.
**Treating Stomach Acid with Antacids: Comfort for Heartburn and Indigestion**
Stomach acid plays a crucial role in digestion, but when it becomes excessive, it can lead to discomfort and ailments like heartburn and indigestion. Enter antacids, the heroes that come to the rescue by neutralizing stomach acid and providing much-needed relief.
Antacids are bases that act as a neutralizing force, countering the acidic environment of the stomach. When taken, they react with stomach acid, resulting in the formation of water and salt. This neutralization reaction effectively reduces acidity, making the stomach contents less corrosive and alleviating the burning sensation associated with heartburn.
The benefits of antacids extend beyond heartburn relief. They also provide comfort from indigestion, a common condition characterized by discomfort or pain in the upper abdomen. Indigestion is often caused by an excess of stomach acid, which antacids can effectively neutralize.
Antacids come in various forms, including tablets, liquids, and powders. They are generally safe and well-tolerated, making them a readily-available solution for occasional stomach discomfort. However, it’s important to note that excessive use of antacids can lead to side effects, so it’s essential to use them as directed and consult a healthcare professional if symptoms persist.
Neutralizing Sulfuric Acid: The Role of Weak Acids
In the realm of chemistry, acids and bases engage in a fascinating dance of neutralization, creating a balance that is essential for myriad processes. Among the many substances that can neutralize acids, weak acids play a crucial role, particularly in the context of sulfuric acid.
When a weak acid encounters sulfuric acid, a formidable strong acid, a remarkable transformation occurs. The weak acid, such as acetic acid or citric acid, acts as a soothing balm, reducing the acidity of the sulfuric acid. This neutralization process involves an exchange of protons, where the weak acid donates protons to the sulfuric acid.
As the protons are transferred, the resultant mixture gradually loses its acidic nature. The sulfuric acid, once a corrosive threat, becomes less potent, and its ability to cause harm is diminished. In essence, the weak acid acts as a chemical mediator, reducing the severity of the sulfuric acid’s corrosive properties.
The neutralization process between a weak acid and sulfuric acid also leads to the formation of water and salt. Water, a ubiquitous molecule, serves as a byproduct of the reaction, while salt, a compound composed of positive and negative ions, is the other outcome. These products are relatively harmless and pose no significant threat to the environment or human health.
Benefits of Using Weak Acids for Neutralization
The use of weak acids for neutralizing sulfuric acid offers several advantages.
-
Controlled Neutralization: Weak acids neutralize sulfuric acid at a slower rate compared to strong bases. This controlled neutralization process allows for precise adjustments, ensuring that the desired pH level is achieved without overshooting or undershooting.
-
Reduced Heat Generation: Unlike strong bases, weak acids generate less heat during the neutralization process. This reduced heat generation minimizes the risk of hazardous reactions and ensures a safer environment for handling and storage.
-
Environmental Friendliness: Weak acids are typically derived from natural sources, making them environmentally friendly alternatives to strong bases. Their use poses less harm to ecosystems and aquatic life.
Weak acids play a vital role in neutralizing sulfuric acid, reducing its acidity and corrosive properties. The controlled nature of the neutralization process, reduced heat generation, and environmental friendliness make weak acids a preferred choice in various applications, including industrial settings, laboratories, and water treatment facilities. Understanding the role of weak acids in neutralization is essential for comprehending the delicate balance between acids and bases, a fundamental aspect of chemical reactions.
Performing a Neutralization Reaction: A Chemical Tango
In the realm of chemistry, a neutralization reaction unfolds like an elegant dance between two opposing forces: acids and bases. When these combatants meet, a fascinating transformation takes place, resulting in the creation of new substances with significantly different properties.
Imagine you have a mischievous acid, its molecules bristling with hydrogen ions, ready to donate their acidic bite to anything in their path. Enter a graceful base, its molecules generously carrying hydroxide ions, eager to embrace the hydrogen ions. As they collide, a remarkable exchange occurs.
The hydrogen ions from the acid eagerly join forces with the hydroxide ions from the base, forming water molecules. These water molecules, like tiny dancers, twirl and pirouette, creating a neutral environment. Meanwhile, the remaining ions from the acid and base pair up, forming a salt. This salt is typically a solid substance, much less reactive than its parent acid or base.
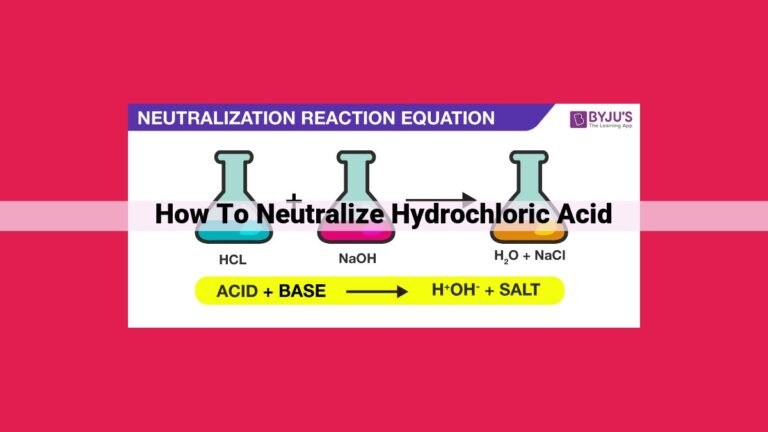
The neutralization reaction can be represented by a chemical equation:
Acid + Base → Water + Salt
For example, when hydrochloric acid (HCl) encounters sodium hydroxide (NaOH), they engage in a chemical waltz, resulting in the formation of water (H2O) and sodium chloride (NaCl), the common table salt we sprinkle on our food.
Steps of a Neutralization Reaction
- Reactant Preparation: Gather your acid and base solutions.
- Mixing: Carefully combine the acid and base solutions in a suitable container.
- Ion Exchange: Hydrogen ions from the acid transfer to hydroxide ions from the base, forming water molecules.
- Salt Formation: The remaining ions from the acid and base combine to form a salt.
- Completion: The reaction continues until all the hydrogen ions have reacted with hydroxide ions.
Implications of Neutralization
Neutralization reactions play a crucial role in numerous chemical processes, both in nature and in industrial applications. From neutralizing stomach acid to treating environmental pollution, this chemical dance has far-reaching implications. So, next time you witness a chemical reaction between an acid and a base, appreciate the enchanting transformation that takes place, creating new substances and altering the chemical landscape around you.
Identifying the Equivalence Point with a pH Indicator
Neutralization reactions, where an acid and a base react to form a salt and water, play a crucial role in many chemical processes. To determine when a neutralization reaction has reached completion, chemists utilize a handy tool known as a pH indicator.
pH indicators are substances that change color depending on the acidity or basicity of a solution. In the context of neutralization reactions, pH indicators are selected that change color at or around the equivalence point, the point where the moles of acid and base are equal.
When an acid and a base are mixed, the solution’s acidity or basicity changes gradually as the reaction progresses. A pH indicator is added to the solution, and as the equivalence point is approached, the indicator undergoes a color change. This color change signals that the solution has reached neutrality, indicating the equivalence point of the reaction.
For instance, phenolphthalein, a common pH indicator, turns colorless in acidic solutions but changes to a vibrant pink in basic solutions. When phenolphthalein is added to an acid solution being neutralized by a base, it remains colorless until the equivalence point is reached. At this point, the solution turns a faint pink, indicating that the acid has been completely neutralized.
pH indicators are essential for precisely determining the endpoint of neutralization reactions, ensuring that the correct amount of base has been added to neutralize the acid. They provide a visual cue for chemists, allowing them to monitor the progress of the reaction and accurately determine when the equivalence point has been reached.
Conducting a Titration to Reach Equivalence
Titration: The Precise Measure of Neutralization
In the world of chemistry, titration shines as a technique used to precisely determine the exact amount of base required to neutralize an acid. Imagine a culinary master meticulously measuring ingredients to create the perfect dish; titration is the chemist’s equivalent, ensuring a harmonious balance between acids and bases.
The Process: Step by Step
Titration involves a carefully controlled experiment where a base solution is gradually added to an acid solution. But here’s the clever part: as the base is added, the pH of the solution is continuously monitored. The pH value serves as a telltale sign, indicating the progress of the neutralization reaction.
Once the equivalence point is reached, the solution reaches neutral pH, signaling that the acid and base have neutralized each other completely. At this point, the amount of base added is precisely the amount required to neutralize the acid. It’s like a chemical handshake where the two reactants come together in perfect balance.
The Equivalence Point: A Colorful Signal
To detect the equivalence point, chemists employ pH indicators, clever substances that change color depending on the pH of the solution. As the titration progresses, the indicator monitors the pH, changing color at the exact moment when neutralization is complete. It’s akin to a traffic light, signaling the transition from acidic to neutral.
The Titration Endgame
Reaching the equivalence point is the goal of titration, and it’s a journey marked by precise measurements and careful observations. By understanding the process and the significance of the equivalence point, chemists gain the power to precisely control neutralization reactions, ensuring a balanced and harmonious chemical world.