Sulfur Oxidation Numbers: Understanding Chemical Bonding, Redox Reactions, And Compound Behavior
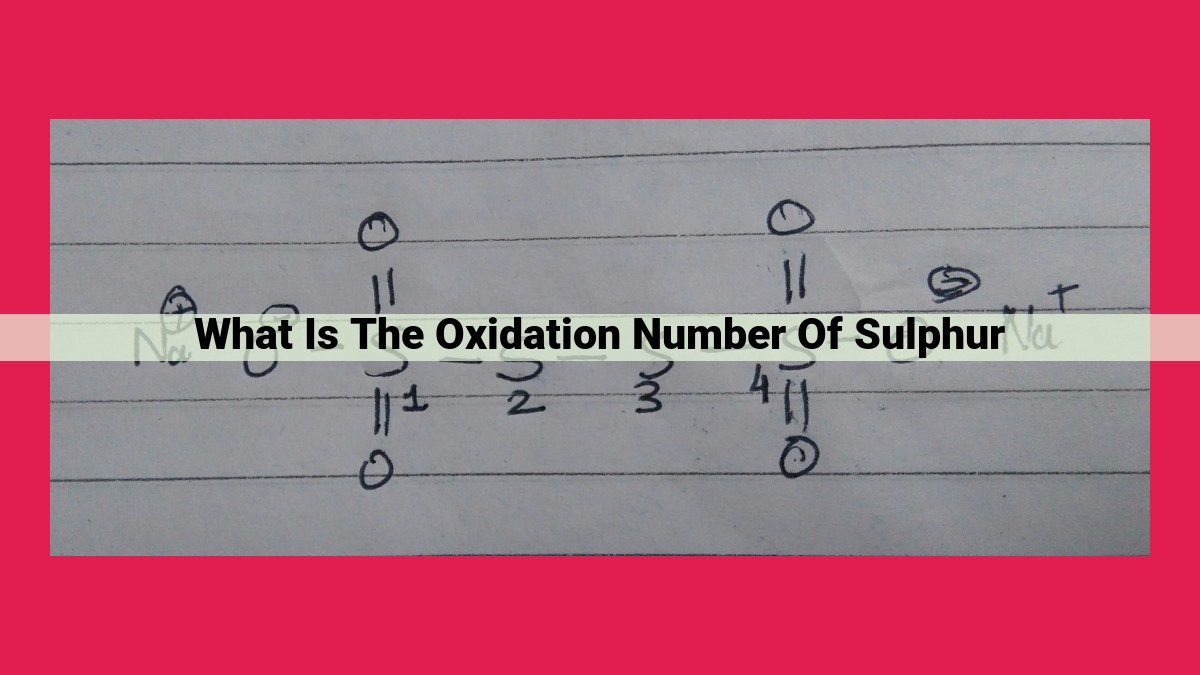
The oxidation number of sulfur represents the hypothetical charge it would have if all its bonds were purely ionic. Sulfur exhibits multiple oxidation states due to its ability to share or accept electrons. In sulfuric acid, sulfur has an oxidation number of +6, forming covalent bonds with oxygen. In sulphates, the oxidation number is +4, while in metal sulphides, it’s -2. Understanding oxidation numbers is crucial for identifying and predicting redox reactions and comprehending the chemical bonding and behavior of sulfur in various compounds.
- Define oxidation number and explain its concept.
- Discuss the role of oxidation number in identifying and predicting redox reactions.
- Highlight the relationship between chemical bonding and oxidation number.
Oxidation Number and Redox Reactions: Unlocking the Secrets of Chemical Transformations
In the realm of chemistry, oxidation number plays a pivotal role in understanding the intricate dance of chemical transformations. It’s a concept that unveils the secrets behind how atoms change their electronic configuration, leading to the formation of new substances with unique properties.
Defining Oxidation Number
Oxidation number assigns a numerical value to each atom in a molecule or ion, representing its relative charge. It reflects the number of electrons that an atom has gained or lost compared to its neutral state. A positive oxidation number indicates that the atom has lost electrons, while a negative oxidation number indicates that it has gained electrons.
Unveiling Redox Reactions
Oxidation number is a powerful tool for identifying and predicting redox reactions, where one species undergoes oxidation (loss of electrons) and another undergoes reduction (gain of electrons). By comparing the oxidation numbers of the atoms before and after the reaction, we can determine which species has been oxidized and which has been reduced. This insight is crucial for understanding a wide range of chemical processes, from respiration to combustion.
Chemical Bonding and Oxidation Number
The relationship between chemical bonding and oxidation number is closely intertwined. Oxidation number reflects the number of electrons that an atom shares with its neighboring atoms. In covalent bonds, electrons are shared equally, resulting in oxidation numbers of zero. In ionic bonds, one atom donates electrons to another, leading to oxidation numbers that reflect the charge of the ions.
Embark on a Deeper Dive into Sulphur
Sulphur, an element renowned for its versatility, exemplifies the concept of oxidation number. It possesses multiple allotropic forms and can exhibit a wide range of oxidation states, from -2 to +6. Understanding the factors that govern sulphur’s oxidation number is essential for comprehending its diverse chemical behavior.
Sulphur: A Versatile Element with Multiple Oxidation States
Sulphur, an alluring element with a diverse repertoire of allotropic forms, has captivated the scientific community for centuries. This enigmatic element exhibits a remarkable ability to adopt various oxidation states, ranging from -2 to +6, unveiling its multifaceted nature.
Allotropic Forms of Sulphur
Sulphur manifests itself in an array of allotropic forms, each adorned with unique structural characteristics. Rhombic sulphur, the most stable form under ambient conditions, boasts an intricate crystalline lattice, reminiscent of an intricate puzzle. Monoclinic sulphur, on the other hand, assumes a needle-like morphology, adding to the element’s diverse visual appeal.
The Origin of Multiple Oxidation States
Sulphur’s unique electronic configuration, featuring six valence electrons, bestows upon it the ability to participate in a wide range of chemical bonding interactions. This versatility, coupled with its ability to form stable covalent bonds with various elements, explains its remarkable proclivity for adopting multiple oxidation states.
Sulphur in Action: Oxidation States in Practice
In the realm of inorganic chemistry, sulphur’s oxidation states dance across the spectrum, showcasing its adaptability. As a component of sulphuric acid, sulphur assumes a lofty +6 oxidation state, unleashing its potency as a corrosive and dehydrating agent. In sulphates, it settles into a more moderate +4 oxidation state, forming stable ionic bonds with metals. Conversely, in metal sulphides, sulphur embraces a -2 oxidation state, forging robust covalent bonds with metals, exemplifying its diverse bonding capabilities.
Oxidation Number of Sulphur: A Deeper Dive
- Define the oxidation number of sulphur and provide examples to illustrate its different values.
- Explain the factors that influence the oxidation number of sulphur in different compounds.
Oxidation Number of Sulphur: A Deeper Dive
Unlocking the intricate world of oxidation numbers, let’s dive deeper into the enigmatic element sulphur. Oxidation number refers to the hypothetical charge an atom would have if the electrons were completely transferred to the more electronegative atoms it’s bonded with. This concept plays a crucial role in understanding the chemistry of sulphur and its diverse compounds.
Sulphur, with its eight valence electrons, exhibits a remarkable range of oxidation states, from -2 to +6. These oxidation numbers reflect the ability of sulphur to share or accept electrons, depending on the elements it interacts with.
Factors Influencing Oxidation Number
The oxidation number of sulphur is influenced by several factors:
- Electronegativity: Sulphur has an electronegativity of 2.58, making it more electronegative than most metals but less electronegative than oxygen and halogens.
- Bonding Type: The type of bond between sulphur and other atoms affects the oxidation number. In ionic compounds, sulphur tends to have a negative oxidation number, while in covalent compounds, it can have positive, negative, or zero oxidation numbers.
- Molecular Structure: The molecular structure and geometry of a compound can influence the oxidation number of sulphur. For example, in sulphuric acid, sulphur exhibits an oxidation number of +6 due to its double bonds with oxygen.
Examples of Sulphur Oxidation States
- -2: Metal sulphides (e.g., FeS)
- 0: Elemental sulphur (e.g., S8)
- +4: Sulphates (e.g., CaSO4)
- +6: Sulphuric acid (e.g., H2SO4)
Understanding the oxidation number of sulphur provides valuable insights into the chemistry of this versatile element. It enables chemists to predict the reactivity of sulphur compounds, identify redox reactions, and design new materials with tailored properties.
Oxidation Number +6: Sulphuric Acid
- Describe sulphuric acid, its properties, and applications.
- Explain the role of sulphur in the formation of sulphuric acid, focusing on its high oxidation state (+6).
Sulphuric Acid: A Powerhouse with Sulphur at Its Peak
In the realm of chemistry, oxidation number plays a crucial role in understanding and predicting chemical reactions. Among the elements that showcase this concept is sulphur, a versatile chameleon capable of assuming multiple oxidation states. One of its most remarkable avatars is in sulphuric acid, where sulphur reigns supreme with an oxidation number of +6.
Sulphuric acid (H2SO4) stands as a cornerstone of modern chemistry and industry. Its corrosiveness, dehydrating properties, and exceptional acidity make it a formidable reagent in a myriad of applications. From fertilizers to batteries, detergents to dyes, sulphuric acid’s versatility is unmatched.
At the heart of sulphuric acid lies sulphur, the element with a diverse range of allotropes (elemental forms). Its ability to exist in various oxidation states reflects its remarkable chemical adaptability. In sulphuric acid, sulphur’s high oxidation number (+6) reflects its strong oxidizing power.
The formation of sulphuric acid involves the oxidation of sulphur dioxide (SO2), a toxic gas released during the combustion of fossil fuels. Through a complex process involving oxygen and water, sulphur dioxide is transformed into sulphur trioxide (SO3), which reacts with water to form sulphuric acid.
Sulphur’s role in this reaction is paramount. Its high oxidation state (+6) drives the oxidation of sulphur dioxide, enabling the formation of sulphuric acid. This process underscores the importance of oxidation number in understanding the chemical transformations that shape our world.
In the grand tapestry of chemistry, sulphuric acid stands as a towering figure, showcasing the power of sulphur and the significance of oxidation number. Its versatility and chemical prowess make it an indispensable tool, while its unique formation mechanism highlights the interplay between elements and their oxidation states.
Oxidation Number +4: Sulphates
- Define sulphates and provide examples of common sulphate compounds.
- Explain the bonding between sulphur and oxygen in sulphates and how it affects the oxidation number (+4).
Oxidation Number +4: Sulphates
Sulphates are ionic compounds that contain the sulphate ion, which is composed of a sulphur atom bonded to four oxygen atoms. The sulphate ion has a charge of -2, and it is commonly found in combination with positively charged metal ions. Some of the most common sulphate compounds include sodium sulphate, potassium sulphate, and calcium sulphate.
Sulphates are formed when sulphur reacts with oxygen in the presence of a metal. The exact mechanism of the reaction depends on the specific metal involved, but in general, the sulphur atom is oxidized to a +4 oxidation state, while the oxygen atoms are reduced to a -2 oxidation state. The metal ion then forms an ionic bond with the sulphate ion to create the sulphate compound.
The bonding between sulphur and oxygen in sulphates is covalent. Each sulphur atom is bonded to four oxygen atoms by single bonds. The oxygen atoms are arranged in a tetrahedral shape around the sulphur atom. This tetrahedral arrangement is the most stable configuration for the sulphate ion, and it is responsible for the ion’s high symmetry and low reactivity.
The oxidation number of sulphur in sulphates is +4. This means that the sulphur atom has lost four electrons in forming the sulphate ion. The oxidation number of sulphur can be determined by using the following rules:
- The oxidation number of an element in its pure form is 0.
- The oxidation number of an element in a monatomic ion is equal to the charge of the ion.
- The sum of the oxidation numbers of all the atoms in a neutral compound is 0.
- The sum of the oxidation numbers of all the atoms in a polyatomic ion is equal to the charge of the ion.
Using these rules, we can determine that the oxidation number of sulphur in sulphates is +4. This is because the sulphate ion has a charge of -2, and the oxygen atoms each have an oxidation number of -2. Therefore, the oxidation number of sulphur must be +4 in order for the sum of the oxidation numbers to be 0.
Metal Sulphides: Intriguing Compounds with Oxidation Number -2
In the realm of oxidation numbers, metal sulphides stand as fascinating entities that exhibit a unique oxidation state of sulphur: -2. These compounds, formed by the union of metals and sulphur, possess remarkable properties and play pivotal roles in various chemical processes.
Formation and Properties of Metal Sulphides
Metal sulphides often form when metals react with sulphur or hydrogen sulphide gas. These reactions frequently produce exothermic compounds, characterized by high stability and low solubility in water. The diverse colors and appearances of metal sulphides further add to their appeal.
Sulphur’s Role in Bonding and Oxidation Number
In metal sulphides, sulphur atoms acquire an oxidation number of -2. This distinctive oxidation state arises from covalent bonding between sulphur and the metal atoms. The sulphur atom contributes two valence electrons to the covalent bond, forming stable and nonpolar molecules.
Applications of Metal Sulphides
Metal sulphides boast a wide range of applications in various industries. Iron pyrites, for instance, are used as sources of sulphur and iron. Copper pyrites serve as ore minerals for extracting copper. Additionally, metal sulphides have found uses in electrochemistry, semiconductors, and photography.
Metal sulphides are captivating compounds that illustrate the diverse oxidation states of sulphur. Their distinctive oxidation number of -2, stemming from covalent bonding with metals, grants them intriguing properties and valuable applications across numerous fields. These remarkable compounds continue to inspire further research and exploration within the fascinating realm of chemistry.