Essential Subatomic Particles Of The Atom: Protons, Neutrons, And Electrons
Essential Particles of an Atom
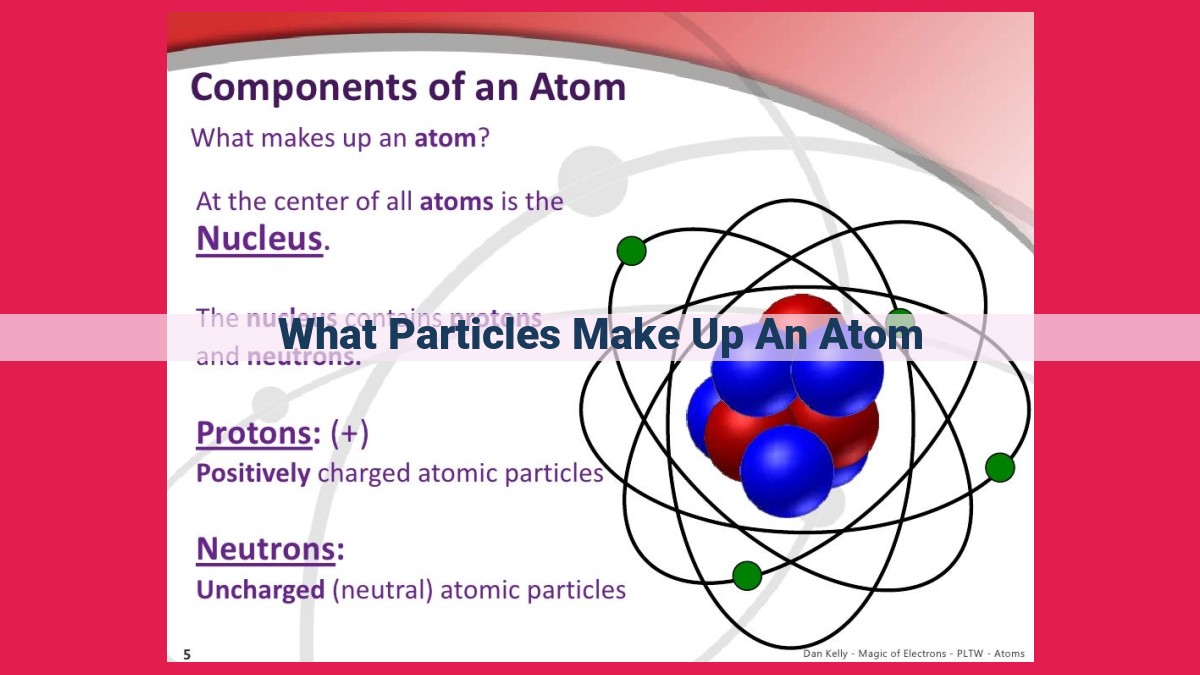
An atom consists of two types of subatomic particles: nucleons (protons and neutrons) and electrons. Nucleons reside in the atom’s nucleus, with positively charged protons defining the element’s identity and uncharged neutrons contributing to its mass. Electrons, negatively charged particles, orbit the nucleus in energy levels. The atomic number represents the number of protons, determining the element’s type, while the mass number encompasses both protons and neutrons, indicating the atom’s mass.
Essential Particles of an Atom
Nucleons: The Building Blocks of the Nucleus
In the heart of every atom lies the nucleus, a densely packed bundle of subatomic particles called nucleons. Nucleons are the atomic realm’s fundamental building blocks, the essential ingredients that define an atom’s identity.
Two types of nucleons exist: protons and neutrons. Protons, the positively charged counterparts, are responsible for an element’s atomic number, the unique identifier that distinguishes one element from another. Neutrons, on the other hand, are uncharged particles that contribute to an atom’s mass.
Protons and neutrons are held together within the nucleus by a powerful force known as the strong nuclear force. This force overcomes the electrostatic repulsion between the positively charged protons, ensuring the nucleus’s stability.
The number of protons in the nucleus determines the atomic number, which in turn determines the element’s chemical properties. For instance, all atoms with one proton are hydrogen, while those with six protons are carbon.
The total number of nucleons, including both protons and neutrons, determines the mass number of an element. Mass number plays a crucial role in determining an atom’s mass and its position on the Periodic Table.
Understanding the composition of nucleons is essential for unraveling the mysteries of the atomic world. These fundamental particles form the very essence of matter, defining the identity and properties of every element in the universe.
Protons: The Positively Charged Heartbeats of Elements
In the heart of every atom, a tiny, densely packed core known as the nucleus holds the secrets to an element’s identity. Within this minuscule realm, amidst a sea of particles, protons reign supreme.
Protons are the positively charged particles that define an element. Their presence, or absence, determines the unique characteristics that distinguish one element from another. Each proton carries a single positive charge, balancing out the negative charges of electrons that orbit the nucleus.
The number of protons in an atom’s nucleus is known as its atomic number. This number, like a blueprint, dictates the element’s position on the periodic table and its fundamental properties. For instance, hydrogen has one proton, while oxygen boasts eight. This difference in proton count profoundly influences the elements’ chemical behaviors and physical characteristics.
Protons play a crucial role in defining an element’s reactive nature. They determine the number of electrons that the atom can accommodate in its outer electron shells. This electronic arrangement, in turn, governs the element’s ability to form bonds with other atoms. Without protons, atoms would lose their individuality and the tapestry of life as we know it would unravel.
So, there you have it. Protons, the positively charged heartbeats of elements, are the architects of atomic identity. They determine an element’s unique traits and lay the foundation for the intricate dance of chemical reactions that shape our world.
Unveiling the Secrets of Neutrons: The Silent Contributors to Atomic Mass
In the heart of every atom lies a mysterious world of subatomic particles, including the enigmatic neutrons. These unassuming particles may not carry an electrical charge, but they play a vital role in shaping the identity and behavior of elements. Let’s embark on a journey to unravel the secrets of neutrons and their profound influence on the atomic realm.
Neutrons reside within the nucleus, the tiny, dense core of an atom. They share this space with protons, the positively charged particles that determine the element’s identity. Unlike protons, neutrons are neutral in charge, which means they don’t participate in chemical reactions. However, their presence is far from insignificant.
Neutrons contribute significantly to the overall mass of an atom. Each neutron adds a single unit of mass, known as an atomic mass unit (amu). The total number of protons and neutrons in an atom determines its mass number, which is expressed as an integer.
Impact on Mass Number
The mass number is a crucial factor in identifying isotopes, which are atoms of the same element that differ in their neutron count. For example, carbon-12 has six protons and six neutrons, giving it a mass number of 12. In contrast, carbon-13 has six protons and seven neutrons, resulting in a mass number of 13.
The greater the number of neutrons in an atom, the heavier it will be. This is because neutrons are roughly the same mass as protons, but they lack a positive charge that would cancel out some of the negative charge from electrons.
Maintaining Nuclear Stability
Neutrons also play a key role in maintaining the stability of the nucleus. Protons repel each other due to their positive charges. However, neutrons, being neutral, can act as a “buffer” between protons, reducing the repulsive forces and keeping the nucleus intact.
In conclusion, neutrons, though uncharged, are indispensable components of an atom. They contribute to the mass of an atom and influence its mass number, which is essential for distinguishing between isotopes. Moreover, their presence in the nucleus provides stability, preventing the protons from flying apart due to their mutual repulsion. Without neutrons, the atomic world would be a much different place, and the elements we know would not exist in their familiar forms.
Essential Particles of an Atom: An Atomic Odyssey
In the unfathomable depths of our atomic realm, where the very essence of matter unfolds, a symphony of particles dances in harmonious coexistence. Join us on an expedition to unravel the secrets of these fundamental building blocks, unravelling the mysteries of the atom.
Nucleons: Building Blocks of the Nucleus
At the heart of every atom, a bustling metropolis known as the nucleus plays host to nucleons, the powerhouses that define its identity. These subatomic denizens come in two flavours: protons and neutrons. Protons, the knights of the atomic realm, carry a positive charge, while neutrons, their enigmatic counterparts, remain neutral. Together, they form the bedrock upon which the atom rests.
Protons: Positively Charged Heartbeats
Protons are the gatekeepers of an element’s identity. Their number, known as the atomic number, determines the element’s place in the periodic table. These positively charged particles orchestrate the atom’s symphony, dictating its chemical properties.
Neutrons: Mass Contributors
In the shadows of the protons reside neutrons, the silent giants that augment the atom’s mass. Their presence, while not altering its charge, shapes the atom’s mass number, a testament to its atomic weight.
Electrons: Orbiting Negatives
Now, let us venture beyond the nucleus to the ethereal realm of electrons, negatively charged particles that pirouette around the nucleus in a graceful dance. These cosmic ballet dancers are not nucleons, but their presence is equally vital to the atom’s equilibrium.
Electrons are the architects of chemical reactions, their interactions with other atoms governing the boundless complexities of our world. Their orbits, like celestial bodies, define the atom’s size and shape, creating the foundation for the myriad forms matter can assume.
Atomic Nucleus: The Atom’s Core
- Define the atomic nucleus as the central part of an atom.
- Highlight that it contains the nucleons (protons and neutrons).
Essential Particles of an Atom
At the heart of every atom, there lies a tiny, dense realm known as the nucleus. This microscopic core holds the very blueprint of an element’s identity. Within its depths reside two fundamental particles: protons and neutrons.
Protons are the positively charged building blocks of the nucleus. They are responsible for defining an element’s atomic number, which dictates its unique position on the periodic table. The number of protons within an atom is a constant, providing a fingerprint-like identification for each element.
Neutrons, unlike protons, are uncharged particles that reside alongside them in the nucleus. They contribute significantly to an atom’s overall mass but play no role in determining its atomic number. The presence of neutrons influences the mass number of an element, which represents the total number of nucleons (protons and neutrons) within the nucleus.
Together, protons and neutrons form the atomic nucleus, the central powerhouse that holds the atom together. The nucleus is incredibly small, occupying only about 10^-15% of the atom’s volume. Despite its diminutive size, it contains the vast majority of the atom’s mass, as electrons contribute negligible weight.
The atomic nucleus is a remarkable testament to the intricate balance and harmony of nature. The positive charge of protons is precisely counteracted by the equal and opposite negative charge of electrons orbiting the nucleus, creating an atom that is electrically neutral.
The Building Blocks of Matter: Essential Particles of an Atom
Every physical object in our world, from towering skyscrapers to the smallest speck of dust, is made up of tiny fundamental particles called atoms. These atoms, like intricate LEGO blocks, are constructed from even more elementary particles called nucleons, protons, neutrons, and electrons.
Nucleons: The Nucleus’s Core
At the heart of every atom lies the nucleus, a dense core packed with positively charged protons and uncharged neutrons. These particles, collectively known as nucleons, are responsible for an atom’s mass and identity.
Protons: Positively Charged Heartbeats
Protons are the positively charged particles that define an element’s chemical properties. The number of protons in an atom determines its identity and its place on the periodic table. Each element has a unique number of protons, giving it its characteristic atomic number.
Neutrons: Mass Contributors
Neutrons, on the other hand, are neutral particles that contribute to an atom’s mass but do not affect its chemical behavior. The number of neutrons, along with the number of protons, determines the mass number of an element.
Electrons: Orbiting Negatives
Surrounding the nucleus like a swarm of tiny planets orbit the electrons, negatively charged particles that are responsible for chemical reactions and electrical conductivity. Electrons do not contribute significantly to an atom’s mass and are not considered nucleons.
Atomic Nucleus: The Atom’s Core
The atomic nucleus is the dense, central part of an atom that houses the protons and neutrons. It is extremely small compared to the overall size of the atom, yet it contains nearly all of its mass.
Atomic Number: Unique Element Identity
The atomic number of an element is an integer that uniquely identifies it on the periodic table. It corresponds to the number of protons in the nucleus and determines the element’s chemical characteristics. Each element has a distinct atomic number, distinguishing it from all other elements.
Essential Particles of an Atom: Understanding the Building Blocks
Every atom, the fundamental unit of matter, is composed of a nucleus surrounded by electrons. The nucleus, the core of the atom, contains positively charged protons and neutral neutrons, collectively known as nucleons.
Protons, with their positive charge, are responsible for an element’s atomic number, which uniquely identifies each element on the periodic table. Neutrons, on the other hand, have no charge and primarily contribute to an atom’s mass. The sum of protons and neutrons in an atom’s nucleus determines its mass number.
Mass Number: Counting Nucleons
The mass number of an atom represents the total number of nucleons present in its nucleus. It is essentially the sum of the number of protons and neutrons within the atom. Since protons and neutrons have approximately the same mass, the mass number provides a convenient way to estimate the mass of an atom.
The mass number, denoted by A, is crucial in understanding the isotopic variations of an element. Isotopes are atoms of the same element that have the same atomic number but different mass numbers. This variation in mass number indicates that isotopes have the same number of protons but different numbers of neutrons. For example, carbon has three stable isotopes: carbon-12 (A = 12), carbon-13 (A = 13), and carbon-14 (A = 14), each with different numbers of neutrons.
Understanding the mass number of an atom is fundamental to various scientific disciplines, including chemistry and physics. It helps scientists determine the relative abundance of isotopes in nature, study nuclear reactions, and predict the properties of different elements and compounds.