Understanding The Structure And Function Of Phospholipids: Essential Components Of Biological Membranes
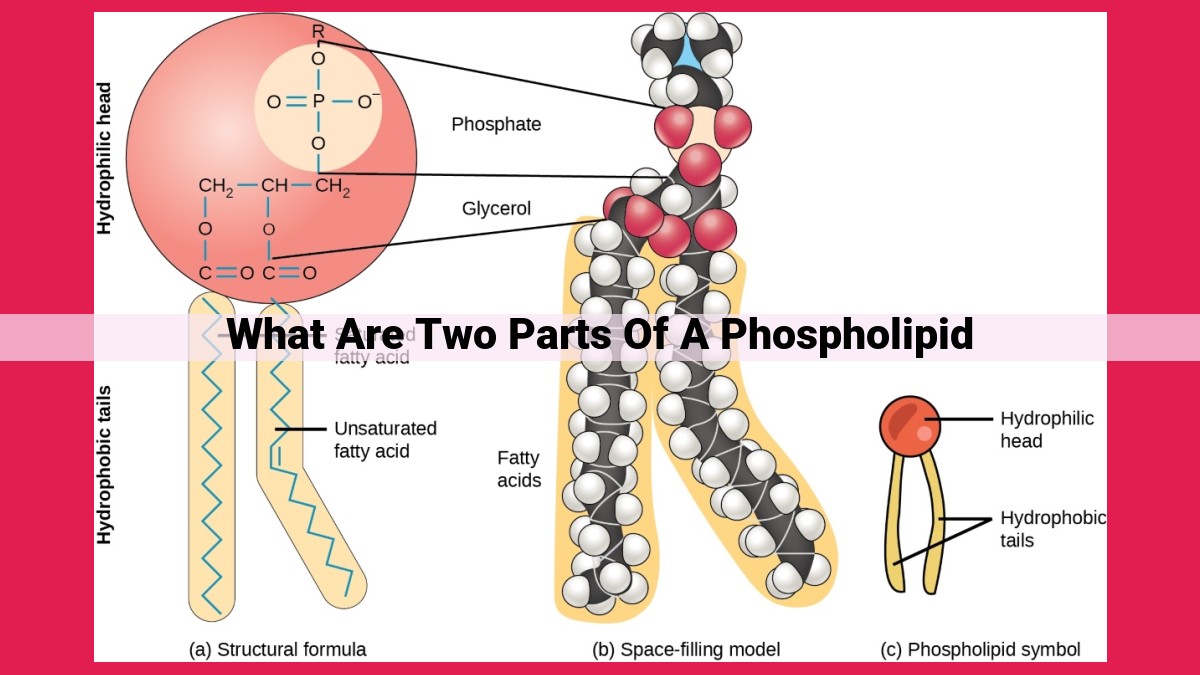
Phospholipids, crucial components of biological membranes, consist of two distinct parts: a hydrophilic head featuring a phosphate group and glycerol, attracting water molecules; and a hydrophobic tail composed of fatty acid chains, repelling water. This amphipathic nature allows phospholipids to form the lipid bilayer, with hydrophilic heads facing the aqueous environment and hydrophobic tails shielded within the membrane’s core.
The Heartbeat of Cells: Uncovering the Essential Components of Biological Membranes
Biological membranes, the bustling frontiers of our cells, play an irreplaceable role in life’s grand symphony. They act as gatekeepers, regulating the flow of materials into and out of cells. Their intricate structure and unique components enable them to perform this vital task with remarkable precision.
The mainstay of biological membranes is phospholipids, a class of molecules possessing the remarkable ability to self-assemble into a double layer. This lipid bilayer is the foundation upon which the membrane’s myriad functions are built.
To fully appreciate the brilliance of these membranes, let’s delve into the captivating world of their constituent components, starting with the hydrophilic head.
Dive into the World of Biological Membranes: Unveiling the Hydrophilic Head
In the bustling realm of biology, cellular membranes play a crucial role as the gatekeepers of our cells, regulating the passage of substances and protecting their precious contents. These membranes are composed of a complex symphony of molecular players, with phospholipids taking center stage as their primary building blocks.
A phospholipid molecule is a tale of two contrasting worlds. Its hydrophilic head revels in the company of water molecules, while its hydrophobic tail shuns them like a plague. This unique duality is what enables phospholipids to form the lipid bilayer, the fundamental structure of biological membranes.
The hydrophilic head, adorned with a phosphate group and a glycerol molecule, has an inherent affinity for water. It’s like a social butterfly, attracting water molecules and forming a cozy embrace around the membrane’s outer layer. This hydrated shell ensures that the membrane remains in constant contact with its watery environment, facilitating the exchange of substances between the cell and its surroundings.
Moreover, the hydrophilic heads are responsible for the membrane’s fluidity. They engage in a graceful dance with water molecules, allowing the membrane to adjust its shape and respond to changing conditions. This fluidity is essential for a cell’s dynamic processes, such as endocytosis and exocytosis.
The hydrophilic head, therefore, is not merely a passive structure but an active player in the membrane’s symphony. It orchestrates the membrane’s interactions with water, maintaining fluidity, and ultimately safeguarding the cell’s delicate balance.
Diving Deeper into the Hydrophilic Head: A Membrane’s Water-Loving Gateway
In the intricate realm of biological membranes, the hydrophilic head reigns as a water-loving guide, orchestrating the membrane’s harmonious interplay with its surroundings. Composed of a phosphate group and a glycerol molecule, this polar structure eagerly embraces water molecules, forming the outer layer of the lipid bilayer – a membrane’s foundation.
This hydrophilic head is more than just a bystander; it actively influences membrane fluidity, the ease with which the membrane can flow and adapt. As water molecules dance around these hydrophilic heads, they create a lubricating effect, allowing the membrane to flex and bend in response to cellular demands.
Furthermore, the hydrophilic head’s polar nature is the cornerstone of phospholipid’s amphipathic character, the ability to simultaneously love and repel water. This remarkable duality gives membranes their unique ability to separate the watery interior of the cell from its external environment, a crucial barrier for maintaining cellular integrity and function.
Diving into the Water-Repelling Nature of the Hydrophobic Tail
The journey through the enigmatic world of phospholipids continues as we dive deeper into the hydrophobic tail, an integral component that forms the heart of biological membranes. Unlike its hydrophilic counterpart, the hydrophobic tail shuns water and embraces its repelling nature.
Structure and Arrangement: A Fortress of Nonpolarity
The hydrophobic tail consists of fatty acid chains, which are long carbon-based molecules with a nonpolar nature. This nonpolarity arises from the absence of charged or polar groups that would attract water molecules. As a result, the hydrophobic tails aggregate, forming a solid and fluid-restricting core within the lipid bilayer. This core effectively shields the cell’s interior from the aqueous environment, providing a crucial barrier against leakage and external threats.
Impact on Membrane Fluidity: A Delicate Balance
The hydrophobic tail plays a pivotal role in modulating the fluidity of biological membranes. Fatty acid chain length significantly influences fluidity. Longer chains pack tightly, increasing rigidity and reducing fluidity. In contrast, shorter chains allow for more flexibility. Saturated fatty acid chains, with no double bonds, further enhance rigidity, while unsaturated fatty acid chains with double bonds create kinks and promote fluidity. This delicate balance is crucial for cellular processes, as membranes need to be fluid enough to allow for molecule exchange yet rigid enough to maintain cell integrity.
In conclusion, the hydrophobic tail of phospholipids forms a water-repellent and fluid-regulating core of biological membranes. Its nonpolar nature and interactions with fatty acid chain length and saturation shape membrane properties, underscoring the intricate symphony played by phospholipids in maintaining cellular health and function.
The Hydrophobic Tail: Shaping Membrane Dynamics
Delve into the hydrophobic core of the lipid bilayer, a crucial barrier that shields the cell from its aqueous surroundings. The nonpolar nature of the hydrophobic tails, composed of fatty acid chains, repels water and forms a protective layer within the membrane.
Imagine the hydrophobic core as a fortress, safeguarding the cell from the harsh external environment. Its impermeability ensures that essential molecules remain safely within the cell, while harmful substances are kept at bay.
Furthermore, the composition and interactions of the hydrophobic tails significantly influence membrane fluidity. Shorter, saturated fatty acid chains promote a more rigid membrane, while longer, unsaturated chains increase fluidity. This delicate balance is crucial for maintaining optimal membrane function, including nutrient transport and cell signaling.
The hydrophobic tails also contribute to the amphipathic nature of phospholipids. Amphipathic molecules possess both hydrophilic (water-loving) and hydrophobic (water-repelling) properties. This duality allows phospholipids to self-assemble into the lipid bilayer structure, with hydrophilic heads facing outward toward the aqueous environment and hydrophobic tails facing inward, creating a semi-permeable barrier.