Starch: A Vital Macromolecule In Plant Metabolism And Beyond
Starch is a macromolecule classified as a polysaccharide, a complex carbohydrate composed of multiple glucose units. As a plant’s energy reservoir, starch plays a vital role in plant metabolism. Its two main components, amylose and amylopectin, differ in structure and properties. Amylose consists of linear glucose chains, while amylopectin has branched chains. Similar to starch structure, glycogen serves as an energy storage molecule in animals. Starch remains a crucial component in both natural and industrial processes, highlighting its significance as a macromolecule.
Understanding Macromolecules and Their Vital Role in Life
Imagine a vast city filled with countless buildings, each serving a unique purpose in maintaining the city’s function. In the realm of biology, macromolecules play a similar role within living organisms. They are colossal molecules, essential building blocks that form the very foundation of life.
Macromolecules are complex structures composed of thousands or even millions of smaller molecules called monomers. These giant molecules are responsible for a mind-boggling array of biological processes, from storing energy to transporting genetic information. Without macromolecules, life as we know it would not exist.
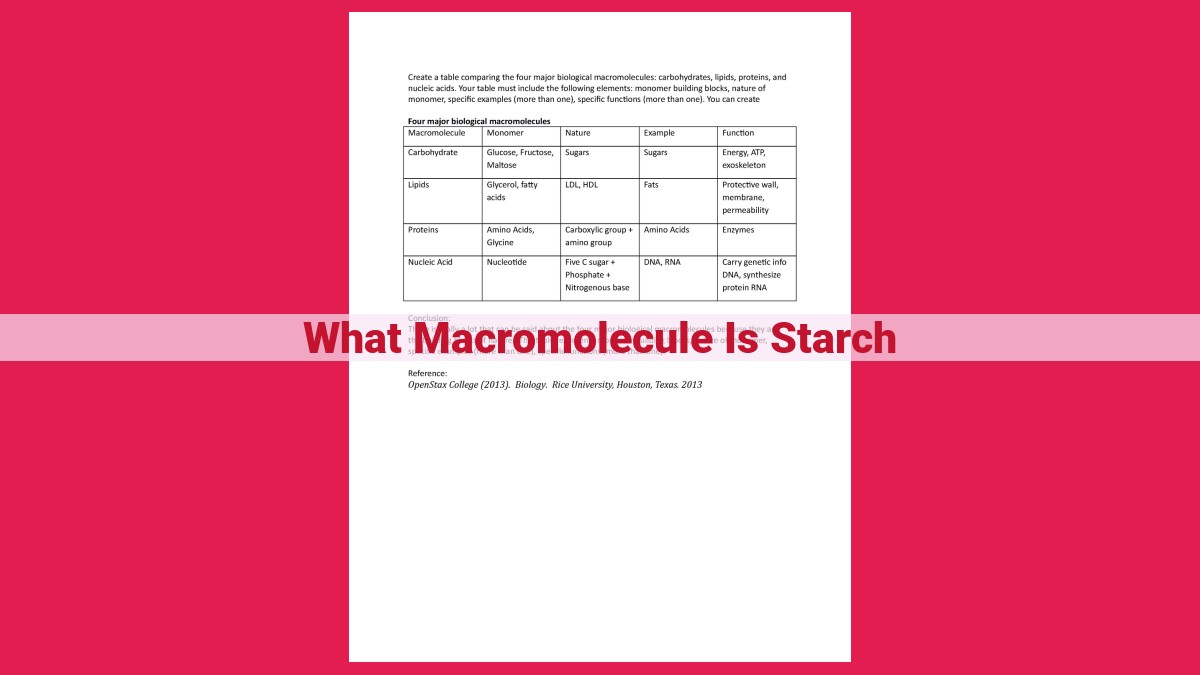
Carbohydrates: The Building Blocks:
- Explain the definition of carbohydrates and their classification into simple and complex types.
Carbohydrates: The Building Blocks of Life
In the vast realm of biochemistry, macromolecules reign supreme, shaping the fabric of living organisms. Among these giants, carbohydrates stand out as the foundational blocks, providing energy and structure to all living beings.
Carbohydrates, composed of carbon, hydrogen, and oxygen, come in two broad categories: simple and complex. Simple carbohydrates, also known as sugars, are the sweet-tasting molecules that provide a quick burst of energy. They include glucose, fructose, and galactose, found in fruits, honey, and dairy products.
In contrast, complex carbohydrates are large, intricate structures that serve as a more sustained source of energy. They are known as polysaccharides, meaning “many sugars.” These include starch, cellulose, and glycogen.
Starch, found in plants, is the primary energy storage macromolecule. It consists of numerous glucose units, linked together in a complex array. Cellulose, the main structural component of plant cell walls, is an indigestible fiber that provides support and rigidity to plants. Glycogen, present in animals and humans, is a highly branched polysaccharide that serves as a reserve energy source for muscles.
Polysaccharides: The Complex Carbohydrate Structures
Polysaccharides, intricate macromolecules, are the epitome of carbohydrate complexity. These giants of the carbohydrate family are composed of intertwined chains of monosaccharides, simpler sugars that serve as the building blocks of carbohydrates.
Starch, a ubiquitous polysaccharide in plant cells, holds a prominent place as a reservoir of energy. Its role in plant metabolism is vital, storing excess glucose units for later use. Cellulose, another polysaccharide, is the backbone of plant cell walls, providing structural support and rigidity.
Glycogen, a polysaccharide found in animal cells, performs a similar function to starch, acting as an energy store. However, glycogen’s structure mirrors amylopectin, a branched component of starch, allowing for quick release of glucose when the body demands energy.
Starch: The Vital Energy Reservoir of Plants
In the world of botany, macromolecules, like intricate building blocks, form the foundation of life. Among these macromolecules, carbohydrates dance to the rhythm of energy, taking center stage as the primary fuel for life’s processes.
Starch, a complex carbohydrate tucked away in plant cells, serves as a fortress of energy storage. Built from countless glucose units woven together like a tapestry, starch presents a safety net for plants during times of need. In the leafy kingdom, starch plays a crucial role in photosynthesis, where sunlight is harnessed to produce glucose, the fuel for life. The excess glucose finds its way into starch, becoming a reserve tank for later use.
The realm of starch is not a monotonous one. Amylose, a starch component, resembles a straight, unyielding rod, while its counterpart, amylopectin, is a labyrinth of branches, its structure akin to a sprawling tree. Together, they form a dynamic duo, amylose lending starch its rigidity and amylopectin providing flexibility.
Amylose and amylopectin, with their unique traits, dictate starch’s properties. Amylose’s rigidity makes starch less digestible, while amylopectin’s branched structure allows for easier breakdown. These properties influence starch’s role in plant metabolism, from energy storage to digestion.
In the tapestry of life, starch is more than just a reserve of energy. It plays a pivotal role in the food industry, thickeners and stabilizers in countless culinary creations. Starch finds its way into bioplastics and biodegradable materials, echoing its significance in the modern world.
Amylose and Amylopectin: Structural Components of Starch:
- Describe the structural differences between amylose and amylopectin, the two components of starch.
- Explain their varying properties and functions.
- Discuss glycogen’s structural resemblance to amylopectin.
Amylose and Amylopectin: The Structural Duo of Starch
Starch, a vital macromolecule in plants, plays a crucial role in energy storage. Comprising monomers called glucose units, starch exists in two distinct forms: amylose and amylopectin. These components vary in their structural intricacies, properties, and functions.
Structural Dissimilarities
Amylose is a linear chain of glucose units connected by α-1,4-glycosidic bonds. Its structure resembles a long, unbranched necklace. In contrast, amylopectin is a highly branched molecule with α-1,4-glycosidic bonds forming the main chain and α-1,6-glycosidic bonds creating the branches. This intricate branching pattern gives amylopectin a tree-like appearance.
Varying Properties and Functions
Amylose forms a tightly packed structure that resists digestion more efficiently than amylopectin. It contributes to starch’s retrogradation, the tendency to crystallize and lose its gelatinous properties upon cooling. Amylopectin, on the other hand, hydrates easily, giving starch its gel-like consistency. Its branches provide ample surfaces for enzymatic action, making it more susceptible to digestion.
Glycogen’s Similarity to Amylopectin
Glycogen, the animal counterpart of starch, bears a striking structural resemblance to amylopectin. Both molecules are highly branched, with α-1,4-glycosidic bonds forming the backbone and α-1,6-glycosidic bonds creating the branches. This similarity in structure reflects their shared role in energy storage.