The Number Of Valence Electrons In Sodium (Na): A Guide To Chemical Bonding
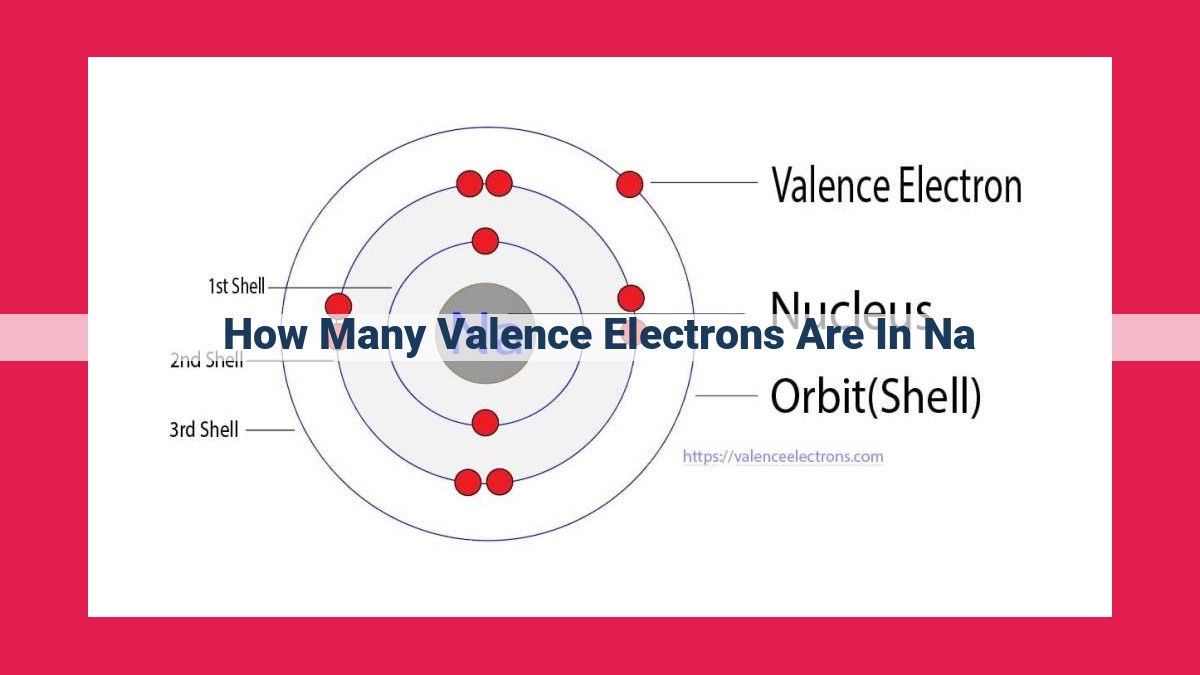
- How Many Valence Electrons in Sodium (Na)
- Valence electrons are the outermost electrons in an atom that participate in chemical bonding.
- Sodium (Na) is an alkali metal with an atomic number of 11, indicating 11 electrons.
- Its electron configuration is 1s22s22p63s1, with one electron in the outermost 3s orbital, making it a monovalent element.
Understanding Valence Electrons: The Key Players in Chemical Reactions
Have you ever wondered why certain elements love to react with each other, while others couldn’t care less? The secret lies in the dance of their valence electrons. These special electrons, perched on the outermost energy level of an atom, hold the key to understanding the chemistry behind these fascinating interactions.
Valence electrons are the electrons that participate in chemical reactions. They determine an element’s reactivity, shape its chemical properties, and dictate how it interacts with other atoms. These dynamic little particles can form bonds with other valence electrons, sharing or transferring them to create new and exciting molecules.
Just as social butterflies crave interaction, elements with a higher number of valence electrons tend to be more reactive. Conversely, those with a solitary valence electron, like introverts, may be less inclined to engage in chemical reactions. So, the number of valence electrons an element possesses gives us clues about its chemical personality.
Electron Configuration: Unveiling the Distribution of Electrons in Atoms
In the realm of chemistry, understanding the behavior of atoms is key to comprehending reactions and properties. Electron configuration plays a pivotal role in this quest, providing a blueprint of how electrons are distributed within atoms.
Imagine an atom as a miniature solar system, with a positively charged nucleus at its core orbited by negatively charged electrons. The electrons, tiny particles, occupy specific energy levels or shells, similar to planets circling a star.
Each shell has a limited capacity for electrons, with the first shell holding a maximum of two, the second shell eight, and so forth. Valence electrons are those found in the outermost shell, and their number and arrangement significantly influence an atom’s chemical behavior.
The electron configuration of an element describes the distribution of its electrons across these energy levels. It’s depicted as a string of numbers, where each number represents the electron count in a particular energy level. For instance, the electron configuration of helium (He) is 1s², indicating that two electrons reside in the first energy level (1s).
This distribution isn’t arbitrary but follows specific rules. Electrons fill the lowest energy levels first and subsequently move to higher energy levels as the atom gains additional electrons. This filling pattern, known as the Aufbau principle, helps predict the electron configuration of various elements.
Electron configuration is a powerful tool for chemists. By analyzing the configuration of a specific element, one can infer its chemical reactivity, bonding behavior, and even its position within the periodic table. Understanding electron configuration is therefore crucial for unraveling the mysteries that lie within the atomic world and paving the way for designing new materials and harnessing chemical reactions.
The Periodic Table: A Guide to Predicting Element Properties
In the realm of chemistry, the periodic table stands as a magnificent tool, illuminating the fundamental properties of elements based on their position. This ingenious arrangement of chemical elements reveals intricate patterns and trends, allowing scientists to predict and understand the behavior of matter with remarkable precision.
Imagine a meticulously organized grid, where elements are meticulously positioned in rows and columns. Each horizontal row, known as a period, represents a specific energy level of electrons. The columns, or groups, denote shared chemical characteristics, giving rise to families of elements with similar properties.
Within each square of this periodic table lies a wealth of information. The atomic number, located at the top of the box, represents the number of protons (positively charged particles) in the nucleus of the element’s atom. This unique identifier distinguishes each element from all others.
Moving to the top right-hand corner, we find the symbol of the element, a one- or two-letter abbreviation that provides a shorthand notation for its name. This concise representation is essential for chemists to communicate and manipulate chemical formulas.
Element Name and Atomic Mass occupy the center of the box, providing further insights into the element’s identity and mass. Atomic mass, measured in atomic mass units (amu), reflects the average mass of the element’s atoms, taking into account the weighted contributions of its isotopes (atoms with the same number of protons but differing numbers of neutrons).
By comprehending the periodic table’s structure and the wealth of information it holds, scientists gain unparalleled insights into the chemical world. It serves as a guide, not only for understanding the behavior of individual elements but also for predicting the properties of undiscovered or synthesized elements, opening up endless possibilities for scientific discovery.
Atomic Number: The Unique Identifier of Elements
In the realm of chemistry, each element possesses an identity card known as the atomic number. Atomic number is the unique number assigned to an element that defines its position on the periodic table. It represents the number of positively charged protons found in the nucleus of an atom of that element.
The atomic number plays a crucial role in distinguishing elements from one another. Each element has a distinct atomic number, which differentiates its identity and properties. For instance, the atomic number of hydrogen is 1, while that of helium is 2. This means that hydrogen atoms have one proton in their nucleus, while helium atoms have two.
This fundamental difference in the atomic number leads to significant variations in the chemical behavior of the elements. Elements with the same atomic number behave similarly, forming the basis of the periodic table’s arrangement. The periodic table groups elements with similar properties into vertical columns called groups, also known as families. Elements within the same group share the same number of valence electrons, which are the electrons in the outermost energy level of an atom.
Comprehending the concept of atomic number is essential in understanding the diversity and uniqueness of the elements. It lays the foundation for exploring the Periodic Table and unraveling the fascinating world of chemical interactions.
Sodium (Na)
- Describe the properties and characteristics of sodium as an alkali metal element.
Meet Sodium: The **Sparkling Alkali Metal**
In the realm of chemistry, we encounter a vibrant cast of elements, each with its unique traits. One such element, sodium (Na), stands out for its dazzling properties and pivotal role in countless chemical reactions.
Sodium belongs to the alkali metals, a family of highly reactive elements that reside in Group 1 of the periodic table. These elements share a peculiar characteristic: they possess a single valence electron in their outermost shell. This lone electron, eager to dance with others, grants alkali metals their distinctive reactivity and silvery-white appearance.
Sodium, with its solitary valence electron, embodies this reactivity to the fullest. Its outermost electron yearns to break free from its atomic embrace and form bonds with neighboring atoms. This eagerness makes sodium an excellent reducing agent, meaning it has a strong tendency to donate this valence electron in chemical reactions.
Valence Electrons in Sodium: A Key to Reactivity
Imagine yourself as a chemist exploring the world of elements. Today, our focus is on sodium (Na), an alkali metal known for its unique properties.
Sodium’s atomic number, 11, reveals that it has 11 electrons orbiting its nucleus. These electrons are arranged in specific energy levels, with the outermost level known as the valence shell.
Valence electrons, like the outermost soldiers in an army, play a crucial role in an atom’s chemical behavior. In the case of sodium, it has one valence electron. This single electron gives sodium its highly reactive nature.
The presence of a single valence electron means that sodium is eager to lose this electron to achieve a stable electron configuration similar to the noble gas neon. By losing this electron, sodium forms a positive ion (Na+) with a full valence shell.
Sodium’s high reactivity makes it an excellent conductor of electricity and a potent reducing agent. It readily reacts with water, releasing hydrogen gas and forming sodium hydroxide, a strong base. This reactivity also makes sodium a valuable component in various industrial processes, including the production of plastics, glass, and pharmaceuticals.
In conclusion, sodium’s single valence electron is a key factor in its remarkable chemical properties. This electron’s ability to be easily lost or shared makes sodium a highly reactive element that finds applications in a wide range of industries.