Understanding Sodium’s Chemical Reactivity: Valence Electrons And Bonding Behavior
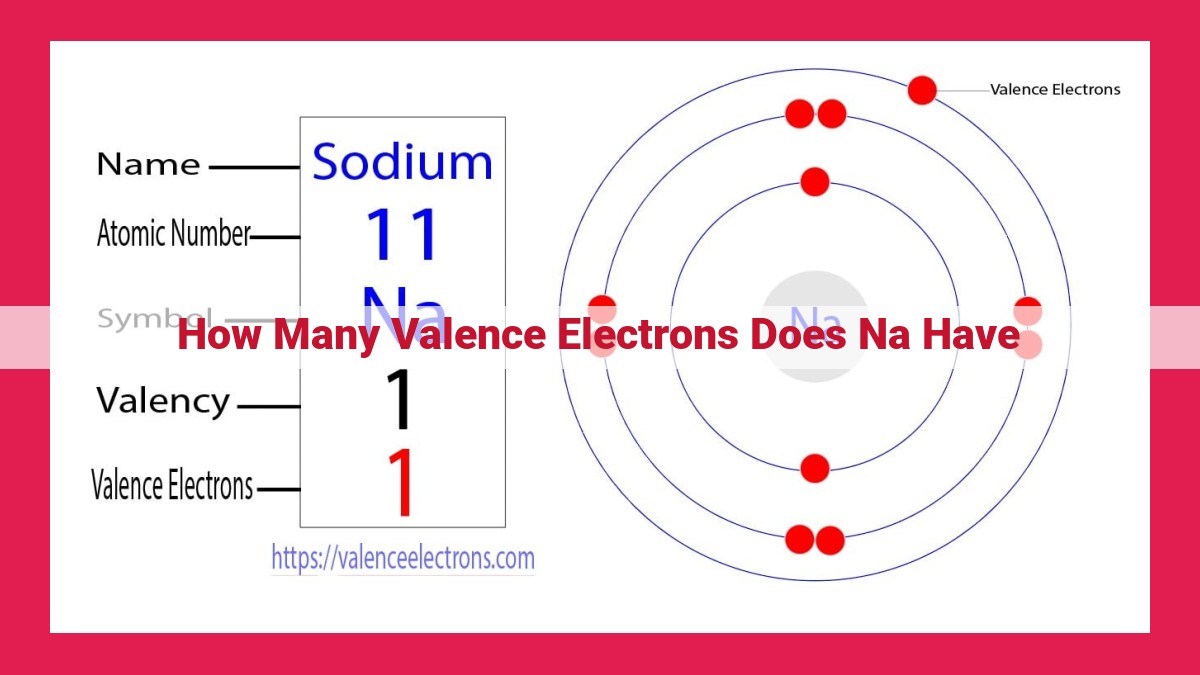
Sodium, with an atomic number of 11, possesses 1 valence electron in its outermost energy level. This is determined by examining its electronic configuration (1s²2s²2p⁶3s¹), where the outermost 3s orbital contains only one electron. Valence electrons play a crucial role in chemical reactions, influencing an element’s bonding behavior. Sodium’s single valence electron makes it chemically reactive, readily forming bonds with other elements to achieve a stable electron configuration.
Understanding the Building Blocks of Sodium: Its Atomic Architecture
In the vast realm of chemistry, elements are the fundamental entities that make up all matter. Each element is characterized by its unique atomic structure, which determines its chemical properties and behavior. Sodium, a highly reactive metal, holds a special place in the world of elements due to its intriguing atomic structure.
An Atomic Odyssey: Exploring the Core
Atoms, the microscopic building blocks of elements, consist of three fundamental particles: protons, neutrons, and electrons. Protons and neutrons reside in the nucleus, the dense center of the atom, while electrons dance around the nucleus in defined energy levels called orbitals.
The Identity Card of Elements: Atomic Number
Each element is assigned a unique atomic number, which represents the number of protons in its nucleus. This number serves as a kind of identity card for elements, differentiating them from one another. Sodium, with an atomic number of 11, possesses 11 protons in its nucleus.
The Importance of Electrons: Beyond the Nucleus
While the nucleus defines an element’s identity, it is the electrons that largely govern its chemical behavior. Electrons arrange themselves in specific orbitals around the nucleus, with each orbital having a fixed energy level. This arrangement, known as the electron configuration, is crucial for understanding an element’s chemical properties.
Sodium’s Electronic Configuration: Unraveling the Language of Chemistry
Imagine embarking on an intergalactic adventure, where atoms are the celestial bodies we encounter. Each atom, like a tiny microcosm, holds a fascinating story within its structure. Today, we delve into the realm of sodium, an element that plays a crucial role in our daily lives.
To fully comprehend sodium’s chemical behavior, we must first explore its electronic configuration. This concept, derived from the principles of quantum mechanics, unveils the arrangement of electrons within an atom.
Electrons, the negatively charged particles that orbit the atom’s nucleus, can be thought of as celestial bodies floating in their own cosmic energy levels. Each level, or orbital, has a specific size and energy. Electrons occupy these orbitals in a manner governed by the Pauli exclusion principle, which states that no two electrons can exist in the same state.
In the case of sodium, its electronic configuration can be represented as 1s²2s²2p⁶3s¹. This cryptic notation tells a compelling story. The 1s² indicates that there are two electrons in the first energy level, occupying the s-orbital. Similarly, the 2s² signifies two electrons in the second energy level’s s-orbital. The 2p⁶ reveals six electrons in the three p-orbitals of the second energy level.
However, the most fascinating aspect lies in the 3s¹. This denotes the presence of a solitary electron in the third energy level’s s-orbital. This lone electron, also known as the valence electron, plays a pivotal role in determining sodium’s chemical properties and its ability to interact with other elements.
Understanding the eletrônico configuration of sodium is like deciphering the secret language of chemistry. It provides us with a deeper understanding of the element’s behavior and sets the stage for exploring its fascinating chemical reactions.
Valence Electrons: The Keystone to Chemical Reactions
In the realm of chemistry, where atoms dance and reactions unfold, understanding the concept of valence electrons is akin to deciphering the secret language of elements. These electrons, residing on the outermost energy level of an atom, hold the key to unlocking an element’s chemical behavior and its ability to forge bonds with other elements.
Valence electrons are like tiny emissaries, eager to interact with their neighbors. They possess an inherent drive to achieve a stable and balanced state, which they pursue by forming chemical bonds. This ceaseless quest for stability dictates the reactions an element undergoes and the compounds it can form.
The number of valence electrons an element possesses directly influences its chemical bonding properties. Elements with a high number of valence electrons, like oxygen or chlorine, exhibit a strong tendency to form ionic bonds, where electrons are transferred between atoms to achieve a full valence shell. Conversely, elements with few valence electrons, such as sodium or potassium, prefer to participate in covalent bonds, where electrons are shared between atoms to create a stable molecular structure.
By comprehending the concept of valence electrons, we gain a profound understanding of the chemical reactivity of elements. It empowers us to predict the types of bonds they will form and the compounds they will create. This knowledge is essential not only for chemists but also for a wide spectrum of scientific disciplines, from materials science to biochemistry.
**Unlocking the Secrets of Sodium’s Valence Electrons**
Embark on a scientific adventure as we delve into the fascinating world of atomic structure and discover the secrets of sodium’s valence electrons. These elusive subatomic particles hold the key to understanding the chemical reactivity and unique properties of this essential element.
Let’s Crack the Atomic Code
Every element in the universe is made up of tiny building blocks called atoms. These atoms are composed of even tinier particles: protons, neutrons, and electrons. Protons and neutrons reside in the atom’s nucleus, while electrons eagerly dance around it in specific energy levels. Each element has a unique atomic number, which determines the number of protons it harbors. Sodium’s atomic number is 11, indicating that its nucleus houses 11 positively charged protons.
Electrons: The Orchestrators of Chemistry
Electrons are the ultimate architects of chemical behavior, and it’s their arrangement within an atom that dictates an element’s reactivity. The electrons in the outermost energy level of an atom, called valence electrons, play a crucial role in determining the element’s bonding properties.
Unveiling Sodium’s Valency
So, how do we determine the number of valence electrons in sodium? It’s all about its electronic configuration. Electronic configuration describes the arrangement of electrons in energy levels, and for sodium, it looks like this: 1s²2s²2p⁶3s¹. This notation tells us that sodium has:
- Two electrons in the first energy level (1s²)
- Two electrons in the second energy level (2s²)
- Six electrons in the third energy level (2p⁶)
- One electron in the fourth energy level (3s¹)
Aha! The outermost energy level (3s) houses only one electron, which means sodium has one valence electron.
The Significance of Sodium’s Lone Electron
This lone valence electron is the driving force behind sodium’s chemical reactivity. It gives sodium the ability to easily form bonds with other atoms, making it an essential component in many chemical processes. Sodium’s valence electron participates in chemical reactions, forming bonds that give rise to the element’s unique properties and the formation of compounds such as sodium chloride (table salt).