Original Title Optimized Title Keywords Striations In Skeletal Muscle Cells Understanding Skeletal Muscle Striations: A Microscopic Journey Skeletal Muscle, Striations, Sarcomere, Thick Filaments, Thin Filaments, A-Bands, I-Bands, H-Zones, M-Lines, Contraction, Light Refraction
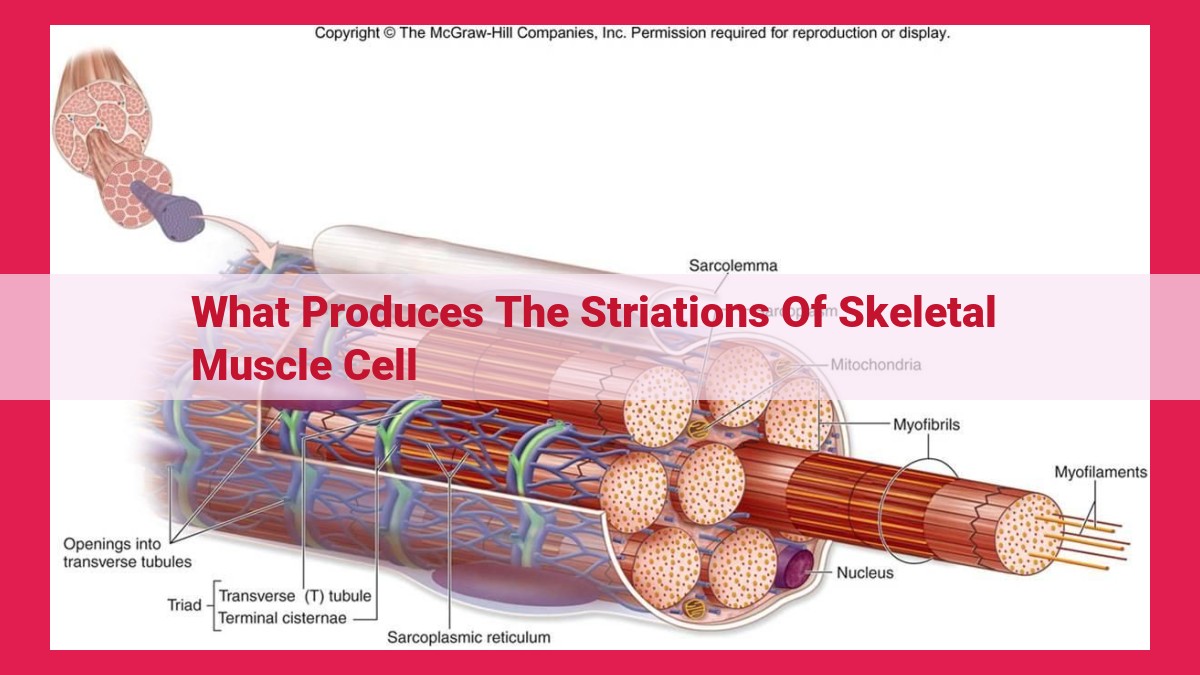
Striations in skeletal muscle cells arise from the organized arrangement of sarcomeres, the basic units of contraction. Sarcomeres comprise alternating thick and thin filaments, anchored by Z-lines and separated by I-bands. Thick filaments, containing myosin, overlap with thin filaments, consisting of actin, in A-bands. H-zones represent areas where thick filaments are absent, while M-lines align them. Filaments slide during contraction, altering H-zone and I-band widths, producing visible striations due to differences in light refraction.
Sarcomeres: The Microscopic Marvels Behind Muscle Striations
Imagine a microscopic world where tiny units, called sarcomeres, come together to form the intricate fabric of our muscles. These sarcomeres, like building blocks, arrange themselves in repeating patterns, creating the characteristic striations that give muscles their unique appearance.
Each sarcomere consists of a multitude of actin and myosin filaments, the fundamental players in muscle contraction. Actin filaments, thin and thread-like, form the scaffolding of the sarcomere, while the thicker myosin filaments slide along them, powering muscle movement.
At the very center of the sarcomere lies the M-line, a dense protein structure that anchors myosin filaments in place. To either side of the M-line, A-bands stretch outward, containing interdigitated actin and myosin filaments. This intricate overlapping gives muscles their characteristic striated appearance.
Flanking the A-bands are the H-zones, regions where only myosin filaments are present. As muscles contract, the H-zones shrink in size, while the I-bands, which are made up exclusively of actin filaments, widen. This dynamic interplay between filaments underlies the mechanism of muscle contraction.
Z-Lines and I-Bands: Defining the Sarcomere Boundaries
- Role of Z-lines in anchoring thin filaments
- Composition and significance of I-bands
Z-Lines and I-Bands: The Pillars of Sarcomere Architecture
Within the intricate tapestry of muscle tissue lies a fascinating world of microscopic units called sarcomeres. These structural wonders, responsible for the characteristic striated appearance of muscle fibers, are precisely organized with distinct regions that define their boundaries. Among these regions, the Z-lines and I-bands play crucial roles in anchoring thin filaments and establishing the overall sarcomere architecture.
Z-Lines: The Anchor Points for Thin Filaments
Imagine a row of tiny rods arranged parallel to each other. The Z-lines are the anchors that hold these rods in place. They are composed of a protein called alpha-actinin, which forms a lattice-like structure that links the ends of thin actin filaments from adjacent sarcomeres. This linkage prevents the filaments from sliding past each other during muscle contraction and relaxation, ensuring the stability and proper alignment of the sarcomere.
I-Bands: The Spaces Between Thin Filaments
Adjacent to the Z-lines lie the I-bands. These regions are composed of thin actin filaments that are not overlapping with thick myosin filaments. During muscle relaxation, the I-bands appear as lighter bands under a microscope due to their reduced protein density. The length of the I-bands decreases during muscle contraction as the thin filaments slide toward the center of the sarcomere, overlapping with the thick filaments.
In summary, the Z-lines act as the anchors for thin actin filaments, while the I-bands represent the spaces between these filaments. Together, these structures define the boundaries of the sarcomere and contribute to the striated appearance of muscle fibers. Understanding their roles is essential for comprehending the intricate mechanisms of muscle contraction and relaxation.
A-Bands, H-Zones, and M-Lines: The Thick Filament Zone
A-Bands: Where Thick and Thin Filaments Interdigitate
At the heart of the sarcomere lies the A-band, an intricate region where thick and thin filaments interlace. These thick filaments are composed of myosin molecules, while the thin filaments consist primarily of actin. The thick filaments appear darker in electron micrographs due to their larger diameter, while the thin filaments are lighterer.
H-Zones: A Tale of Uncovered Myosin
Within the A-band, there exists a mysterious region known as the H-zone. This zone is unique in that it lacks thin filaments, exposing the bare myosin thick filaments. The H-zone’s length varies depending on the muscle’s contraction state, serving as a dynamic indicator of muscle activity.
M-Lines: Guardians of Filament Alignment
At the center of the H-zone, a thin line emerges, known as the M-line. This stabilizing structure is essential for aligning and anchoring the thick filaments, ensuring their synchronized contractions. The M-line’s presence ensures that the thick filaments remain parallel and precisely positioned within the sarcomere.
Filament Interaction and Striation Formation: Unraveling the Secrets of Muscle Movement
When it comes to striated muscles, the secret to their rhythmic contractions lies within the intricate dance of protein filaments. These microscopic structures, known as actin and myosin, orchestrate a symphony of movement that allows our bodies to perform a vast array of actions.
At the heart of this sophisticated machinery lies the sarcomere, the basic unit of muscle contraction. Its alternating bands of light and dark create the distinctive striated pattern that gives these muscles their name. At the boundaries of the sarcomere lie the Z-lines, anchoring the thin actin filaments.
Within the sarcomere, the thick myosin filaments and thin actin filaments interdigitate like fingers in an intricate clasp. When the muscle receives a signal to contract, the myosin heads attach to the actin filaments and undergo a remarkable shape change, pulling the filaments toward the center of the sarcomere. This filament sliding causes the H-zones, the unoverlapped region of thick filaments, to shrink while the I-bands, the region where only thin filaments are present, expand.
As the filaments slide, they alter the light-reflecting properties of the muscle, creating the characteristic striation pattern. When the muscle is fully contracted, the H-zones disappear, and the I-bands become narrower, resulting in a compact arrangement of filaments.
This intricate dance of filament interaction and sliding is made possible by a host of accessory proteins. Titin, a giant protein, provides elasticity to the muscle and ensures that the filaments return to their resting positions after contraction. Tropomyosin and troponin act as gatekeepers, regulating when the myosin heads can interact with the actin filaments.
These molecular mechanisms, from filament sliding to accessory protein regulation, form the foundation of muscle contraction, enabling us to move, breathe, and perform countless other essential functions. Through an understanding of these intricate processes, we gain a deeper appreciation for the incredible complexity and elegance of the human body.
Anisotropy and Other Related Concepts
From the microscopic level, muscles exhibit unique properties that contribute to their remarkable functionality. One such property is anisotropy—the ability of muscle fibers to refract light differently along different axes. This optical phenomenon arises from the organized arrangement of myofibrils, which are the thread-like structures within muscle cells.
Muscle Birefringence
When a ray of light passes through a muscle fiber, it is split into two rays that travel at different speeds. This behavior, known as muscle birefringence, results from the alternating arrangement of thick and thin filaments within myofibrils. The thick myosin filaments have a higher refractive index than the thin actin filaments, causing the light to bend more as it passes through them.
Elasticity Provided by the Titin Filament
Titin is a giant protein that spans the length of the sarcomere, connecting the Z-lines to the M-line. This filament acts as a molecular spring, providing elasticity to the muscle fiber. When the muscle contracts, titin stretches, storing elastic energy, which is released when the muscle relaxes. This energy storage mechanism contributes to the rapid and efficient movements of muscles.
Role of Tropomyosin and Troponin in Muscle Regulation
Tropomyosin and troponin are regulatory proteins that control muscle contraction. Tropomyosin binds to the actin filaments, while troponin binds to tropomyosin. When calcium ions are released from the sarcoplasmic reticulum, they bind to troponin, which undergoes a conformational change. This change causes tropomyosin to shift its position, uncovering the binding sites on actin for myosin heads. This unmasking allows myosin heads to interact with actin, initiating the process of muscle contraction.