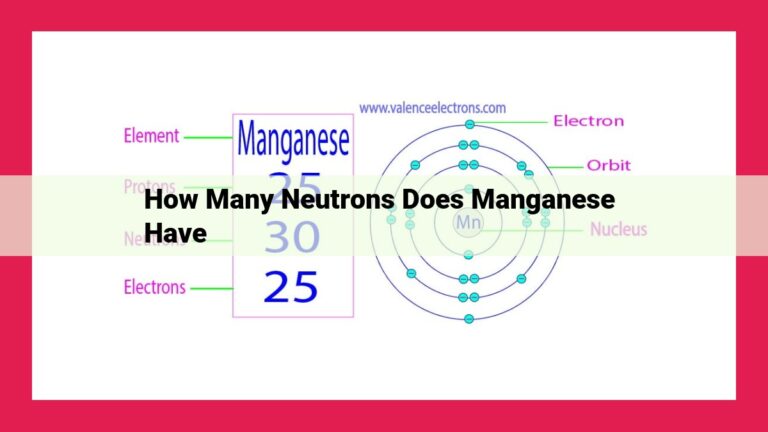
Understanding The Significance Of Proton Count In Atom Identity And Chemical Properties
-
Introduction
The number of protons in an atom is crucial for its identity and chemical properties. The atomic number, which equals the number of protons, defines an element’s position in the periodic table, influencing its chemical behavior and physical characteristics.
The Intriguing Question: How Many Protons Dwell Within an Atom?
Embarking on an enlightening journey into the realm of chemistry, we encounter a fundamental question that sparks our curiosity: How many protons reside within the heart of an atom? This understanding forms the cornerstone of our comprehension of the intricate world of atoms and their interactions.
Unraveling the answer to this enigma holds immense significance for our understanding of basic chemistry. Protons, along with electrons and neutrons, constitute the fundamental building blocks of matter. By grasping the concept of proton count, we gain insights into the identities of elements, their arrangement on the periodic table, and the electrostatic forces that shape atomic interactions. As we delve into the fascinating world of atoms, let’s illuminate the essential role of protons and their profound impact on our understanding of the chemical world.
Atomic Number: The Key to Proton Count
Every atom, the fundamental building block of matter, harbors a tiny nucleus that holds the key to its unique identity. Within this nucleus reside positively charged particles called protons, and it’s their number that determines the atom’s atomic number. This numerical fingerprint not only defines an element but also orchestrates its position in the celebrated periodic table.
Defining Atomic Number
Imagine each element as a character in a grand play, with its atomic number as its unique identification number. This number represents the exact count of protons snugly packed in the atom’s nucleus. As a defining characteristic, the atomic number distinguishes one element from another, creating the diverse cast of elements that make up our world.
Elements Organized by Atomic Number
The periodic table, a masterpiece of chemistry, organizes elements not alphabetically but by their increasing atomic numbers. This ingenious arrangement reveals the fascinating patterns and relationships that govern the elements’ properties. Hydrogen, the simplest element with just one proton, sits at the table’s head, while elements with higher atomic numbers, such as uranium with its 92 protons, occupy the distant realms on the right.
By studying the periodic table, scientists have uncovered a myriad of connections between atomic number and element behavior. For instance, elements with similar atomic numbers often share similar chemical properties. This insight has empowered scientists to predict the properties of newly discovered elements and create materials with tailored functionalities.
Delving into Nuclear Charge
The nucleus, the heart of the atom, carries a positive charge that is directly proportional to the number of protons residing within it. This charge exerts a strong attractive force on the negatively charged electrons that orbit the nucleus, creating the stable structure of the atom.
The delicate balance between nuclear charge and electronic charge is crucial for maintaining atomic stability. An imbalance can lead to the formation of charged particles known as ions, which play a pivotal role in various chemical reactions and biological processes.
The atomic number, an immutable characteristic of each element, provides the foundation for understanding atomic structure and the behavior of elements in the periodic table. By unraveling the secrets of the atomic nucleus and the protons that reside within it, we gain a deeper appreciation for the intricate tapestry of chemistry that weaves the fabric of our world.
Protons: The Positively Charged Subatomic Particles
At the heart of every atom, nestled within the invisible nucleus, lies a fundamental building block known as the proton. These subatomic particles carry a positive electrical charge and play a pivotal role in defining the identity and properties of every element in the universe.
Protons are the guardians of an atom’s core, along with their companion subatomic particles, the neutrons. Together, they form the dense, positively charged nucleus that resides at the center of the atom. This positively charged nucleus serves as the anchor for the surrounding negatively charged electrons, ensuring the atom’s overall neutrality.
The number of protons harbored within an atom’s nucleus holds immense significance. It dictates the atom’s atomic number, a unique identifier that distinguishes it from all other elements. The atomic number, in turn, determines the element’s position within the periodic table, the organizing framework of all known elements.
The Unwavering Connection between Protons and Atomic Number
The atomic number of an element signifies its “identity card” within the realm of chemistry. It represents the specific number of protons found in each atom of that element. For example, hydrogen, the lightest and most basic element, possesses just one proton, giving it an atomic number of 1. On the other hand, gold, a highly prized metal, boasts 79 protons in its nucleus, resulting in an atomic number of 79.
This unwavering bond between an atom’s “proton count” and its atomic number serves as a fundamental principle in understanding atomic structure and the behavior of elements. It governs the chemical properties, reactivity, and interactions that shape the world around us. By comprehending the role of protons in determining an element’s atomic number, we gain a deeper appreciation for the intricacies of the natural world.
The Positive Charge of the Atom’s Nucleus
In the heart of every atom lies a nucleus, a tiny powerhouse brimming with electrical energy. The nucleus is home to protons and neutrons, two fundamental subatomic particles that determine an atom’s identity and behavior. Protons, the positively charged particles, play a pivotal role in the atom’s electrical balance and interactions with its surroundings.
The positive charge of the nucleus, also known as the atomic number, is determined by the number of protons residing within it. Each proton contributes an equal and indivisible unit of positive electrical charge, and the cumulative charge of all the protons creates a positive field surrounding the nucleus.
This positive charge exerts a strong attractive force on negatively charged electrons, which orbit the nucleus in defined energy levels or shells. The counterbalance between the positive nuclear charge and the negative electronic charge creates a stable electrical equilibrium within the atom. This equilibrium is essential for maintaining the atom’s structural integrity and chemical reactivity.
The positive charge of the nucleus also influences the atom’s interactions with other atoms. Atoms with a higher positive charge have a stronger attraction for electrons, making them more likely to form chemical bonds with other atoms. This electrical attraction plays a crucial role in shaping the chemical properties of elements and the formation of molecules and compounds.
Understanding the positive charge of the nucleus is fundamental to comprehending the basic principles of atomic structure and chemistry. It provides insights into the electrical forces that govern the interactions between atoms and molecules, laying the foundation for unraveling the complexities of the chemical world around us.
Atomic Number: The Key to Proton Count
The atomic nucleus, the heart of an atom, harbors a fundamental secret: the number of protons it houses. This pivotal piece of information can be unlocked through a unique identifier, the atomic number. Every element in the vast tapestry of the periodic table possesses a distinct atomic number, a testament to its very essence. This enigmatic number holds the key to not only the number of protons but also the atomic structure and behavior of the element.
The Fundamental Principle
The atomic number of an element is directly proportional to the number of protons residing in its nucleus. This fundamental principle forms the cornerstone of our understanding of atomic structure. The atomic number, a unique and immutable characteristic of each element, dictates the number of protons that an atom of that element possesses.
Implications for Atomic Structure
This relationship has profound implications for comprehending atomic structure. Protons, the positively charged particles within the nucleus, counterbalance the negatively charged electrons that orbit around the nucleus. The delicate interplay between these opposing charges determines the atom’s stability and reactivity. Understanding the atomic number allows us to unravel the intricacies of atomic structure and its impact on chemical behavior.
Examples of Atomic Number and Proton Count
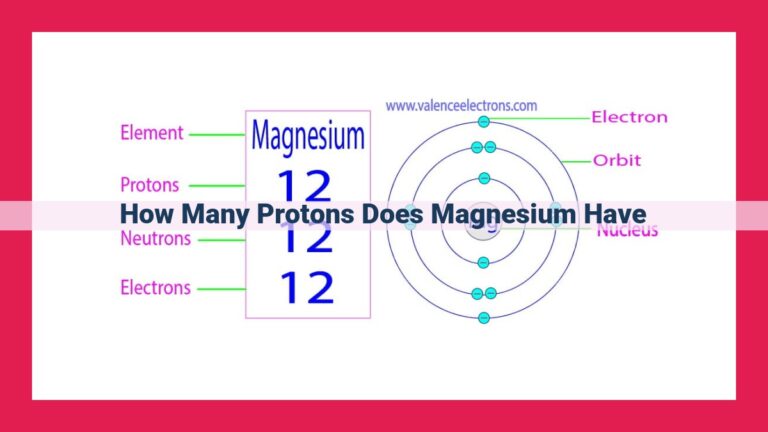
Consider the element hydrogen, the simplest of all, with an atomic number of 1. Each hydrogen atom contains a single proton in its nucleus. In contrast, carbon, the backbone of organic chemistry, boasts an atomic number of 6, indicating the presence of six protons in its nucleus. As we traverse the periodic table, each element’s atomic number progressively increases, reflecting the growing number of protons within their nuclei.
Calculating Protons Using the Periodic Table: Unlocking the Secrets of Atomic Structure
Unveiling the mysteries of atomic structure is an exciting journey that starts with understanding the fundamental components of atoms. One of the most crucial pieces of this puzzle is determining the number of protons in any given atom. Protons, the positively charged particles residing in the heart of an atom, play a pivotal role in shaping its identity and properties.
To unravel this atomic enigma, scientists have devised a powerful tool: the periodic table. This tabular masterpiece offers a treasure trove of information about every known element, including their unique atomic numbers. The atomic number, represented by the symbol Z, is the cornerstone of an element’s individuality, as it reveals the precise number of protons nestled within its nucleus.
Navigating the periodic table to find an element’s atomic number is a straightforward process. Each element resides in a designated box, and nestled within this box, like a precious gemstone, lies the atomic number. For instance, if you seek the atomic number of sodium, simply locate its box in the periodic table. There, you will find the number 11 inscribed within the box, indicating that every sodium atom boasts 11 protons.
This precious piece of information unlocks a wealth of insights into the element’s atomic structure. Protons, with their unwavering positive charge, determine the overall electrical charge of the nucleus. This delicate balance is further complemented by the electrons, negatively charged particles that orbit the nucleus. The interplay between protons and electrons orchestrates the atom’s chemical behavior and reactivity.
Understanding the relationship between atomic number and proton count is pivotal for comprehending the very essence of atoms. It empowers us to predict the behavior of elements, to unravel the intricacies of chemical reactions, and to unlock the secrets of the vast atomic realm.