Understanding Sensible Heat: Key Concepts And Applications
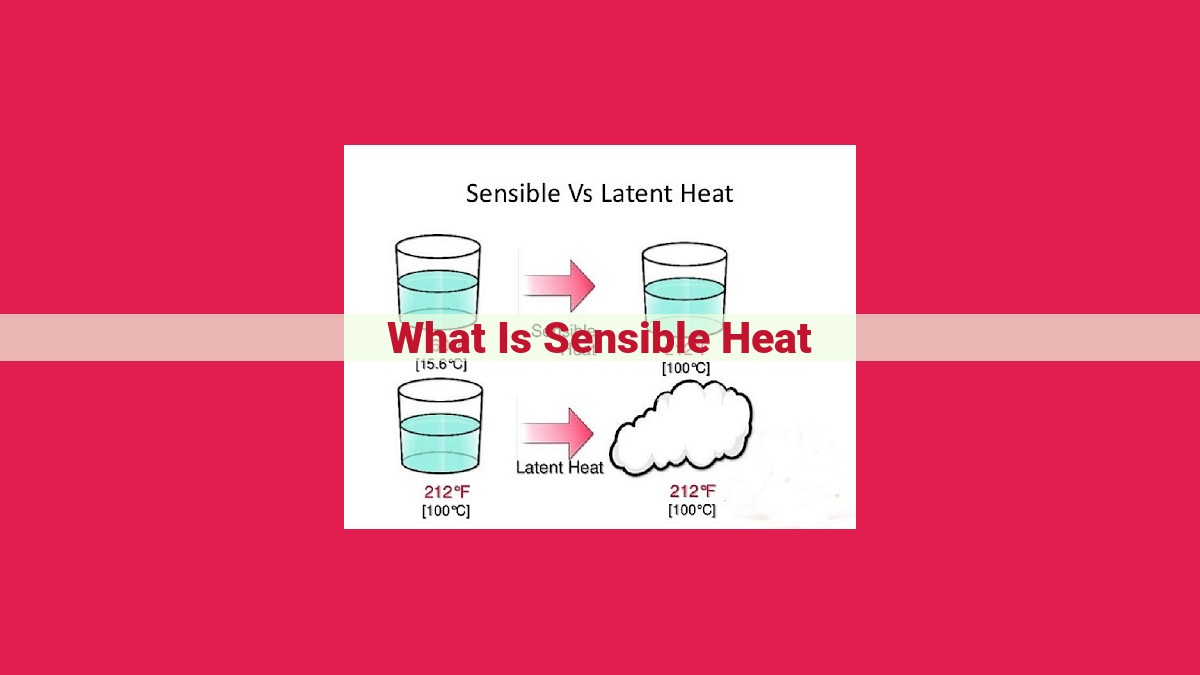
Sensible heat is the heat energy that results in a temperature change of a substance without changing its phase. It is distinct from latent heat, which occurs during phase transitions such as melting or boiling. When heat is added or removed from a substance, its sensible heat changes, resulting in a rise or fall in temperature. This heat transfer occurs due to molecular kinetic energy changes, and the amount of heat absorbed or released depends on the substance’s heat capacity and temperature difference. Sensible heat has significant applications in thermodynamics, engineering, and HVAC systems, as it plays a crucial role in controlling temperature and energy transfer.
Understanding Sensible Heat: A Tale of Temperature Transformation
In the realm of heat exchange, there exists a distinction between two types: sensible heat and latent heat. Sensible heat is the form of heat that causes a temperature change in a substance without altering its phase, such as from solid to liquid or liquid to gas.
Imagine a pot of water on the stove. As the burner heats the water, the molecules gain energy and begin to move faster, increasing their kinetic energy. This increased molecular motion causes the temperature of the water to rise. However, until the water starts to boil and turn into steam, no phase transition occurs.
To illustrate this further, consider two pots of water, each containing the same amount of liquid at the same initial temperature. If one pot is heated over a flame while the other is left at room temperature, the water in the heated pot will gradually become hotter (sensible heat), while the water in the unheated pot will remain at its original temperature.
This difference in temperature between the two pots demonstrates the transfer of sensible heat from the hot pot to the cold pot. As the molecules in the hot water collide with the molecules in the cold water, they transfer some of their energy, causing the temperature of the cold water to increase. This process continues until both pots of water reach the same temperature, at which point the sensible heat transfer stops.
Sensible heat is a crucial concept in the fields of thermodynamics and engineering, where it plays a significant role in heating, cooling, and ventilation systems. Understanding the principles of sensible heat transfer allows us to design and optimize systems that maintain comfortable temperatures in buildings and industrial processes.
Related Concepts:
- Heat Capacity: Importance and specific heat
- Temperature: Measurement of molecular kinetic energy
- Specific Heat: Substance-specific heat capacity
Related Concepts: Unleashing the Essence of Sensible Heat
In our exploration of sensible heat, we encounter a trinity of intertwined concepts that illuminate its fundamental nature. These concepts hold the key to understanding the intricate interplay of heat transfer and temperature change.
1. Heat Capacity: The Unifier of Temperature and Energy
Heat capacity embodies the ability of a substance to absorb and store heat without undergoing a phase transition (such as melting or boiling). It measures the amount of heat required to raise the temperature of a given mass of a substance by 1 degree.
Specific heat, a substance-specific property, quantifies its heat capacity per unit mass. It reveals the unique ability of each substance to absorb and hold thermal energy, underscoring the diversity of the material world.
2. Temperature: A Dance of Molecular Motions
Temperature serves as a measure of the average kinetic energy of molecules within a substance. As molecules collide, their motion becomes more chaotic, manifesting as an increase in temperature. Understanding temperature is crucial for deciphering the flow of heat, as it governs the direction and magnitude of thermal energy transfer.
3. Specific Heat: The Fingerprint of Thermal Affinity
Specific heat unveils the distinctive thermal signature of substances by quantifying their ability to retain heat relative to water. It underscores the substance’s inherent capacity to resist temperature change, providing a window into its molecular structure and composition.
By unraveling the intricacies of these related concepts, we lay the groundwork for a comprehensive understanding of sensible heat. They serve as the building blocks upon which we can explore its mechanisms, influencing factors, and practical applications.
Mechanism of Sensible Heat Transfer
In the realm of thermodynamics, the concept of sensible heat transfer plays a pivotal role. This form of heat exchange involves the transfer of thermal energy between objects of varying temperatures. Unlike latent heat, which involves phase transitions, sensible heat transfer occurs without any change in the physical state of the substance.
The primary mechanism behind sensible heat transfer is the flow of heat from high-temperature objects to low-temperature objects. This flow is driven by the temperature difference between these objects. Imagine a hot cup of coffee placed on a cold table. The heat energy from the coffee will gradually transfer to the table, causing the coffee to cool down while the table warms up.
As the heat transfer progresses, the temperature of the two objects gradually equalizes. This is because the heat energy continues to move from the hotter object (the coffee) to the cooler object (the table) until they reach the same temperature. The process of temperature equalization is crucial in maintaining a stable thermal environment in various applications, such as heating and cooling systems.
It’s important to note that the rate of sensible heat transfer depends on several factors, including the temperature difference, the heat capacity of the objects involved, and the duration of contact. The greater the temperature difference, the higher the heat capacity, and the longer the contact time, the faster the heat transfer will occur.
Factors Influencing Heat Transfer:
- Temperature difference
- Heat capacity of objects
- Duration of contact
Factors Influencing Sensible Heat Transfer
Sensible heat transfer is a fascinating and fundamental concept in thermodynamics. Understanding the factors that influence it is crucial for engineers, scientists, and anyone interested in the efficient transfer of heat. Three key factors play a significant role in determining the rate of heat transfer: temperature difference, heat capacity, and duration of contact.
Temperature Difference:
The temperature difference between two objects or systems is the driving force behind heat transfer. Heat naturally flows from hotter objects to colder objects to achieve thermal equilibrium. The greater the temperature difference, the faster the rate of heat transfer. Imagine a cup of hot coffee placed next to a cold ice cube. The heat from the coffee will transfer to the ice cube, causing it to melt and cool the coffee.
Heat Capacity:
Heat capacity refers to the amount of heat required to raise the temperature of an object by one degree Celsius or Kelvin. Different substances have varying heat capacities. Objects with higher heat capacities require more heat to achieve the same temperature change. For example, water has a higher heat capacity than metal. Therefore, a larger amount of heat is needed to raise the temperature of a pot of water compared to a metal pan.
Duration of Contact:
The duration of contact between two objects or systems affects the rate of heat transfer. The longer the two objects are in contact, the more time there is for heat to flow. For instance, if you place your hand on a warm stove, the longer you keep it there, the more heat will transfer to your hand, potentially causing a burn.
In practical applications, these factors are carefully considered to optimize heat transfer processes. Engineers design heating and cooling systems that maximize temperature differences, utilize substances with high heat capacities, and ensure sufficient contact time between heat exchange surfaces. Understanding these factors is essential for efficient and effective heat management in various industries, from manufacturing to energy production.
Sensible Heat: The Power of Temperature Change
Sensible heat, the concept of heat transfer that doesn’t involve a change in phase, plays a crucial role in our everyday lives. It’s the heat that flows between objects at different temperatures, causing them to reach an equilibrium.
Importance in Thermodynamics and Engineering
Sensible heat is central to thermodynamics, the study of heat and energy. It’s used to calculate the amount of energy required to raise or lower the temperature of a substance. In engineering, it’s essential for designing efficient heating and cooling systems.
Heating, Cooling, and Ventilation Systems
Sensible heat is harnessed in heating, cooling, and ventilation systems to maintain comfortable temperatures in buildings. Air conditioners and refrigerators remove sensible heat to cool down spaces, while heaters add it to warm them up.
For instance, during summer, an air conditioner extracts sensible heat from the indoor air, leaving it cooler. Similarly, in winter, a heater adds sensible heat to the air, making it warmer.
Sensible heat is an essential concept that underlies many aspects of our lives. From regulating temperatures in buildings to powering industrial processes, it’s a fundamental force that shapes our physical world. Understanding sensible heat helps us appreciate the intricate dynamics of heat transfer and its profound impact on our daily experiences.