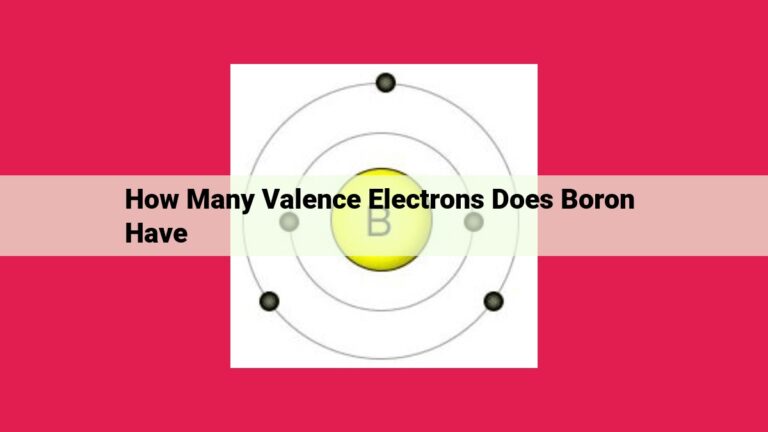
Selenium: The Reactive Chalcogen With 6 Valence Electrons
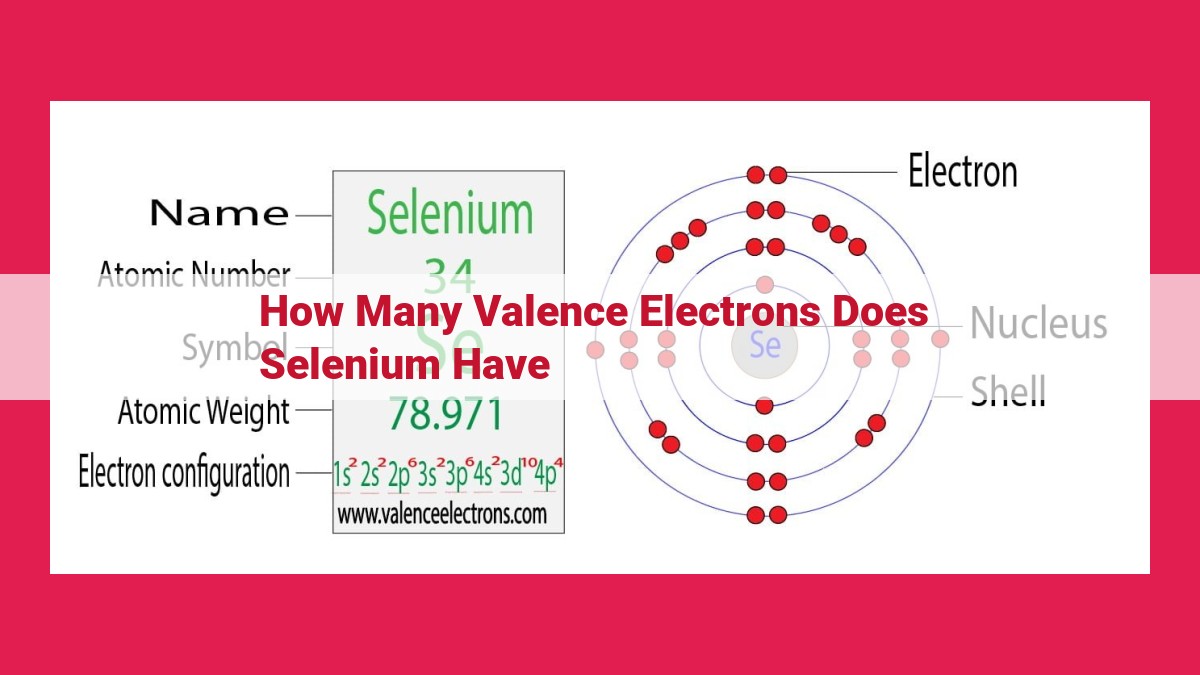
Selenium, a Group 16 chalcogen, has 6 valence electrons. Valence electrons are the outermost electrons in an atom’s electron configuration, determining its chemical reactivity and bonding capabilities. Selenium’s electron configuration, [Ar] 3d10 4s2 4p4, indicates that it has four valence electrons in the 4p orbital. Group 16 elements, known as chalcogens, share the characteristic of having 6 valence electrons, making them highly reactive and prone to forming covalent bonds with other elements.
Valence Electrons: Unlocking the Secrets of Chemical Interactions
Imagine atoms as tiny building blocks, each with a bustling community of electrons orbiting their nucleus. Among this crowd, a special group known as valence electrons plays a crucial role in determining how atoms interact with each other.
Valence electrons are the outermost electrons in an atom’s electron cloud. They are like social butterflies, eager to mingle and form bonds with electrons from neighboring atoms. This bonding behavior, driven by valence electrons, is the foundation of all chemical reactions.
The concept of valence electrons is closely linked to electron configuration, a blueprint that describes how electrons are arranged within an atom. The electron configuration of an element reveals its chemical properties, including the number of valence electrons it possesses.
Electron Configuration: A Map to Valence Electrons
Every element in the periodic table has a unique electron configuration. This configuration, represented by a string of numbers and letters, indicates the number of electrons in each energy level around the atom’s nucleus.
The energy levels, starting from the closest to the nucleus, are labeled “K,” “L,” “M,” and so on. Each energy level can hold a specific number of electrons, like a cosmic parking lot with designated spaces.
Valence electrons reside in the outermost energy level, the one farthest from the nucleus. By examining the electron configuration of an element, we can instantly determine the number of valence electrons it has.
For example, the element sodium has the electron configuration 1s²2s²2p⁶3s¹. The “3s¹” part of this configuration tells us that sodium has one valence electron in its outermost energy level.
Selenium: A Versatile Chalcogen in the Periodic Table
In the vast tapestry of elements that make up our universe, selenium holds a unique position as a Group 16 chalcogen. Nestled within the periodic table, this enigmatic element captivates with its intriguing properties and diverse applications.
Selenium’s home lies in the second period and Group 16 of the periodic table. This placement signifies that it boasts six valence electrons, eagerly waiting to engage in chemical bonding. These valence electrons are the key players in determining selenium’s chemical reactivity and bonding behavior.
As a chalcogen, selenium exudes an amphiphilic nature, meaning it has both hydrophilic (water-loving) and hydrophobic (water-repelling) characteristics. This duality allows selenium to seamlessly interact with both organic and inorganic molecules, making it an adaptable material for a wide range of applications.
Periodic Table and Valence Electrons: Guiding You Through Chemical Bonding
Unlock the secrets of chemistry and gain a fundamental understanding of the Periodic Table, a roadmap to predicting the behavior of valence electrons. These electrons play a pivotal role in shaping the chemical reactivity of elements, forming the foundation for everything from the formation of molecules to the intricate bonds in biological systems.
The Periodic Table is a masterpiece of organization, arranged ingeniously based on atomic number. Each element occupies a specific location, revealing key information about its structure and properties. One of the most crucial insights it provides is the number of valence electrons, which are the outermost electrons in an atom’s electron cloud.
Like a celestial guide, the Periodic Table allows us to effortlessly determine valence electrons. Elements within the same group (vertical column) share a similar number of valence electrons. This pattern empowers us to predict the chemical behavior of an element by simply observing its position on the table.
By deciphering the secrets of valence electrons and their role in the Periodic Table, we embark on a journey into the realm of chemical bonding. These electrons are the key players in chemical reactions, determining an element’s ability to form bonds and shape molecular structures. With this knowledge, we unlock the door to understanding the building blocks of nature and unraveling the secrets of the chemical world.
Valence Electrons and Atomic Number: An Intriguing Correlation
In the realm of chemistry, valence electrons play a pivotal role in understanding the behavior of elements and their ability to form chemical bonds. These electrons reside in the outermost energy level of an atom and determine its reactivity.
One intriguing aspect of valence electrons is their connection to the atomic number of an element. The atomic number represents the number of protons within the atom’s nucleus. This number is unique for each element and dictates its position on the periodic table.
The relationship between atomic number and valence electrons is not coincidental. In fact, the atomic number directly influences the number of valence electrons an element possesses.
To understand this correlation, it’s essential to visualize the structure of an atom. The nucleus, composed of protons and neutrons, resides at the center. Surrounding the nucleus is a cloud of electrons orbiting in various energy levels.
As the atomic number increases, the number of protons in the nucleus also increases. To balance the positive charge of the protons, an equal number of electrons must be present. These electrons fill the energy levels, starting from the lowest.
The outermost energy level, also known as the valence shell, can hold up to eight valence electrons. If an energy level is not filled, the electrons in that level are the valence electrons.
Therefore, by knowing the atomic number of an element, one can predict the number of valence electrons it has. This information is crucial for understanding the chemical properties and reactivity of elements.
Electron Configuration and Valence Electrons
In the realm of chemistry, understanding the behavior of atoms is essential. One key aspect is comprehending valence electrons, the electrons that reside in the outermost shell of an atom and determine its chemical properties.
Electron Configuration Notation
Electron configuration notation is a shorthand way to describe the arrangement of electrons in an atom’s energy levels. It uses numbers and letters to represent the energy levels and the number of electrons in each. For instance, the electron configuration of helium (He) is 1s2, which means it has two electrons in its first energy level (1s).
Valence Electrons in Electron Configuration
Valence electrons are the electrons that occupy the outermost energy level. In electron configuration notation, they are represented by the rightmost number. For helium, the two 1s electrons are its valence electrons.
The number of valence electrons affects an atom’s chemical behavior. Atoms with a full outermost energy level (eight valence electrons) are stable and unreactive, while those with incomplete outermost energy levels tend to react to achieve a stable configuration. For example, sodium (Na) has one valence electron and readily loses it to form stable compounds.
Group 16 Elements and Valence Electrons:
- Describe the common characteristics of Group 16 elements (chalcogens).
- Explain the specific valence electron configuration of Group 16 elements.
Group 16 Elements: The Versatile Chalcogens and Their Valence Electrons
In the realm of chemistry, the Periodic Table stands as a guide to the elements, organizing them based on their atomic number and similarities. Group 16, located on the rightmost column, captivates our attention with its unique set of elements known as the chalcogens. These elements share a common feature: a distinctive valence electron configuration that defines their captivating properties.
Six Valence Electrons: A Shared Characteristic
The secret to the chalcogens’ unity lies in their valence electrons. These are the electrons located in the outermost shell of an atom, and they play a crucial role in chemical bonding. For the chalcogens, the number of valence electrons is six. This remarkable consistency among the group members is a hallmark of their shared identity.
Oxygen, Sulfur, and Selenium: A Trio of Nonmetals
Oxygen, sulfur, and selenium are three prominent chalcogens that exemplify the diverse nature of this group. As nonmetals, they possess a strong electronegativity, meaning they readily attract electrons. This trait makes them excellent at forming chemical bonds with metals and other nonmetals.
Selenium: A Closer Look at Group 16’s Enigma
Among the chalcogens, selenium stands out as a particularly intriguing element. Its electron configuration ([Ar] 3d¹⁰ 4s² 4p⁴) reveals a richness that sets it apart. Four valence electrons reside in selenium’s p-orbitals, granting it a versatile nature that allows it to participate in a wide range of chemical reactions.
Selenium’s Versatility: From Semiconductors to Bioluminescence
Selenium’s unique valence electron configuration contributes to its exceptional properties. It is widely used in semiconductors, where it plays a crucial role in controlling electrical conductivity. Additionally, selenium finds applications in the production of glass, dyes, and pigments. Moreover, its ability to emit light in response to electrical stimulation has made it invaluable in bioluminescent systems.
In conclusion, Group 16 elements, with their consistent valence electron configuration of six, showcase the remarkable power of valence electrons in shaping the chemical behavior of elements. Selenium, with its unique four valence electrons, epitomizes the versatility and fascination of this group. By understanding the role of valence electrons, we gain a deeper appreciation for the diverse properties that make these elements indispensable in the realms of technology, industry, and beyond.
Valence Electrons in Selenium: Unveiling Chemical Bonding Patterns
Selenium, a fascinating element residing in Group 16 of the periodic table, holds a captivating story about its valence electrons. These outermost electrons play a pivotal role in shaping its chemical behavior and unlocking its unique properties.
To delve into the enigmatic world of selenium’s valence electrons, let’s embark on a journey to determine its electron configuration. As we navigate the element’s atomic structure, we discover a total of 34 electrons distributed across energy levels. Particularly noteworthy is the outermost shell, which houses six valence electrons.
These six valence electrons become the architects of selenium’s chemical bonding prowess. They determine how readily the element interacts with others, allowing it to forge bonds with a diverse array of atoms. This versatility makes selenium an indispensable component in a wide range of materials, from semiconductors to photoreceptors.
In the realm of semiconductor applications, selenium’s ability to form both covalent and metallic bonds endows it with exceptional electrical properties. These properties make selenium a crucial ingredient in solar cells, where it converts sunlight into electricity. Moreover, its photoreceptor capabilities render it essential in high-performance imaging devices, capturing and converting light signals into electrical signals.
Unveiling the secrets of selenium’s valence electrons不僅僅是了解元素本身。它揭示了化學鍵的奧秘,打開了一扇通往材料科學和現代科技新發現的大門。了解元素的 valence electrons 就像解鎖一個寶庫,裡面裝滿了創新和進步的可能性。