Uncover The Science Behind Dry Battery Cells: Powering Your Electronic Devices
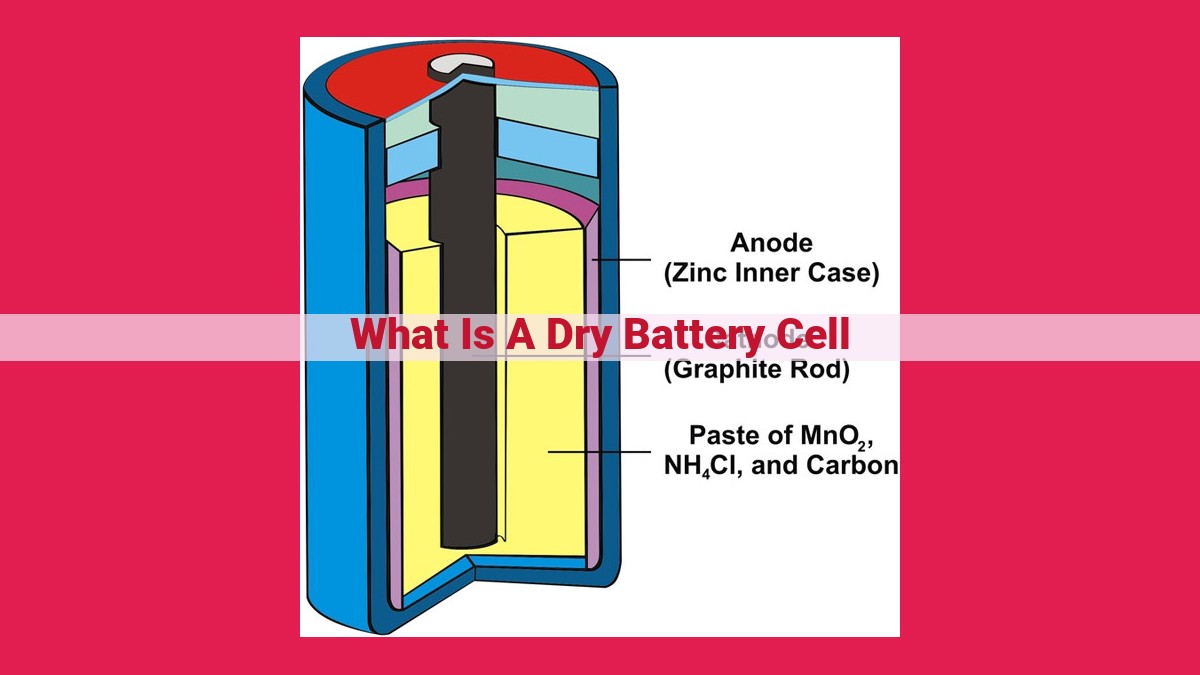
A dry battery cell is an electrochemical device that generates an electrical current through a chemical reaction. It comprises an anode, cathode, and electrolyte paste sealed within a casing. During discharge, an oxidation-reduction reaction occurs, releasing electrons that flow through an external circuit to produce an electric current. Dry batteries provide a constant voltage of 1.5 volts and are commonly used in electronics like flashlights, toys, and portable devices. While they offer advantages such as lightweight and long shelf life, they are not rechargeable and have a limited lifespan.
- Definition and significance of a dry battery cell
- Basic principles of electrochemistry
- Relationship between electrochemical cells and dry battery cells
What is a Dry Battery Cell?
Imagine a tiny powerhouse hidden within our everyday devices, quietly fueling our gadgets and emergency lighting. This is the enigmatic dry battery cell, a marvel of electrochemistry that has revolutionized our lives.
At its core, a dry battery cell is an electrochemical cell that converts chemical energy into electrical energy. It comprises a cathode, an anode, an electrolyte, and a separator. These components work together to generate a steady stream of electrons that power our devices.
Electrochemistry, the science behind dry battery cells, involves the exchange of electrons between two substances. In a dry battery cell, the cathode and anode are made of different materials, each with a unique affinity for electrons. The electrolyte, a gooey paste, provides a path for ion movement and facilitates the chemical reactions that release electrons.
The relationship between electrochemical cells and dry battery cells is intimate. Dry battery cells are a type of electrochemical cell, but they are specifically designed to be compact, lightweight, and have a long shelf life. These attributes make them ideal for portable devices and emergency situations where reliable power is paramount.
Components and Function of a Dry Battery Cell
A dry battery cell, the unsung hero of our everyday lives, is a compact powerhouse that powers a vast array of devices from flashlights to radios. Understanding its inner workings is like peeking behind the curtain of a magic show, revealing the secrets that make it tick.
The Anode: A Zinc-Filled Powerhouse
At the heart of a dry battery cell lies the anode, a zinc cup that serves as a hub for electrons. Within its cavernous depths, zinc atoms, brimming with electrical energy, eagerly await their chance to unleash their power.
The Cathode: A Carbon Conduit of Energy
Opposite the anode, a towering carbon rod stands as the cathode, a conduit for the flow of electrons. Its surface, studded with manganese dioxide, acts as a catalyst, accelerating the chemical reactions that drive the battery’s performance.
The Electrolyte: A Salty, Energy-Rich Paste
Nestled between the anode and cathode is the electrolyte, a salty paste that resembles a conductor filled with electrically charged particles. This viscous substance acts as a bridge, enabling charged particles to travel between the electrodes, carrying the energy that powers the battery.
The Separator: A Silent Guardian of Stability
Keeping the anode and cathode from mingling, like two rival armies, is the separator. This thin, porous barrier prevents direct contact while allowing the electrolyte to flow, ensuring the battery’s efficient and safe operation.
Electrolyte and Chemical Reactions in Dry Battery Cells
The electrolyte in a dry battery cell is not a liquid but a paste made of ammonium chloride and zinc chloride. This paste provides a medium for ion movement within the cell. During the cell’s discharge process, a chemical reaction takes center stage, transforming chemical energy into electrical energy.
The first act of this chemical drama involves zinc atoms from the anode, oxidizing to form positively charged zinc ions (Zn2+). These ions travel through the electrolyte paste towards the cathode. Meanwhile, at the cathode, the triumphant entrance of electrons from the external circuit occurs. These electrons eagerly combine with manganese dioxide (MnO2) and carbon (C), reducing them to form manganese oxide (Mn2O3) and hydroxide ions (OH-).
The flow of electrons from the anode to the cathode creates an electric current, which is the lifeblood of any battery. This process is akin to a symphony of ions, where positively charged zinc ions march towards the cathode, while electrons waltz in the opposite direction. The chemical equation below captures the essence of this electrochemical dance:
Zn + 2MnO2 + 2NH4Cl → ZnCl2 + 2MnO(OH) + 2NH3
In this equation, Zn represents the zinc anode, 2MnO2 the manganese dioxide cathode, and 2NH4Cl the ammonium chloride electrolyte. As the reaction progresses, zinc ions migrate from the anode to the cathode, leaving behind electrons that flow through the external circuit. These electrons then reunite with manganese dioxide and carbon at the cathode, empowering the battery to deliver a continuous flow of electricity.
Voltage and Applications of Dry Battery Cells: Powering Our Devices
Dry battery cells, commonly found in our everyday gadgets, play a crucial role in powering our flashlights, radios, and emergency lighting. The voltage of a dry battery cell is a key characteristic that determines its ability to provide energy to these devices.
Typical Voltage of a Dry Battery Cell
Most dry battery cells have a typical voltage of 1.5 volts. This voltage is generated by the chemical reaction that occurs within the cell. The electrolyte paste in the battery contains positively charged ions and negatively charged ions. When the cell is connected to a circuit, a chemical reaction takes place, causing the positively charged ions to move towards the negative terminal and the negatively charged ions to move towards the positive terminal. This movement of ions creates an electric current, which flows through the circuit and provides power to the device.
Common Applications of Dry Battery Cells
Due to their compact size, lightweight, and long shelf life, dry battery cells are widely used in various devices, including:
- Flashlights: Dry battery cells are the primary power source for flashlights, providing illumination in dark or emergency situations.
- Radios: Portable radios rely on dry battery cells to power their circuitry and allow us to enjoy music or news on the go.
- Emergency Lighting: Dry battery cells are often used in emergency lighting to provide illumination during power outages or other emergencies.
- Wireless Devices: Some wireless devices, such as remote controls and certain toys, utilize dry battery cells as a convenient power source.
Advantages and Disadvantages of Dry Battery Cells
In the realm of portable power, dry battery cells stand out as a reliable and convenient energy source. However, understanding their advantages and disadvantages helps us appreciate their potential and limitations.
Advantages:
-
Lightweight and Compact: Dry battery cells are remarkably lightweight, weighing only a few ounces, and compact in size. This makes them ideal for devices that require portability, such as flashlights, radios, and toys.
-
Long Shelf Life: Unlike perishable foods, dry battery cells boast an impressive shelf life of years, retaining their charge even when stored at room temperature. This extended lifespan ensures their readiness for use when needed, even in emergencies.
Disadvantages:
-
Not Rechargeable: Perhaps the most significant limitation of dry battery cells is their non-rechargeable nature. Once the chemical reaction that generates electricity is complete, the battery cannot be revived. This disposal adds to environmental waste.
-
Limited Lifespan: While dry battery cells have a long shelf life, their actual operational life is limited. Factors like operating conditions, temperature, and device power consumption influence how quickly they discharge. This limited lifespan necessitates frequent replacement, which can be an inconvenience.
Despite their limitations, dry battery cells remain widely used due to their convenience, portability, and affordability. Understanding their advantages and disadvantages empowers consumers to make informed choices and use them efficiently for various applications.
Related Concepts and Applications
In the realm of electrical energy, dry battery cells stand as versatile power sources with a rich history and diverse applications. They share a kinship with their more advanced counterparts, such as lead-acid batteries, lithium-ion batteries, and fuel cells, each with its unique advantages and limitations.
Dry battery cells have found their niche in a myriad of everyday devices. From flashlights that illuminate our paths in the darkness to radios that connect us to the world, these compact energy sources empower us in countless ways. They also serve as the heartbeat of emergency lighting, ensuring that safety is never far from reach.
The voltage of a battery cell, measured in volts, is a crucial factor that determines its performance. A higher voltage indicates a greater potential for electrical work. For instance, a flashlight equipped with a 1.5-volt dry battery cell will emit a brighter beam than one with a lower voltage. However, it’s essential to note that the voltage alone does not define the battery’s capacity or lifespan.