Scandium’s Electron Configuration: Unlocking Chemical Reactivity Through Valence Electrons
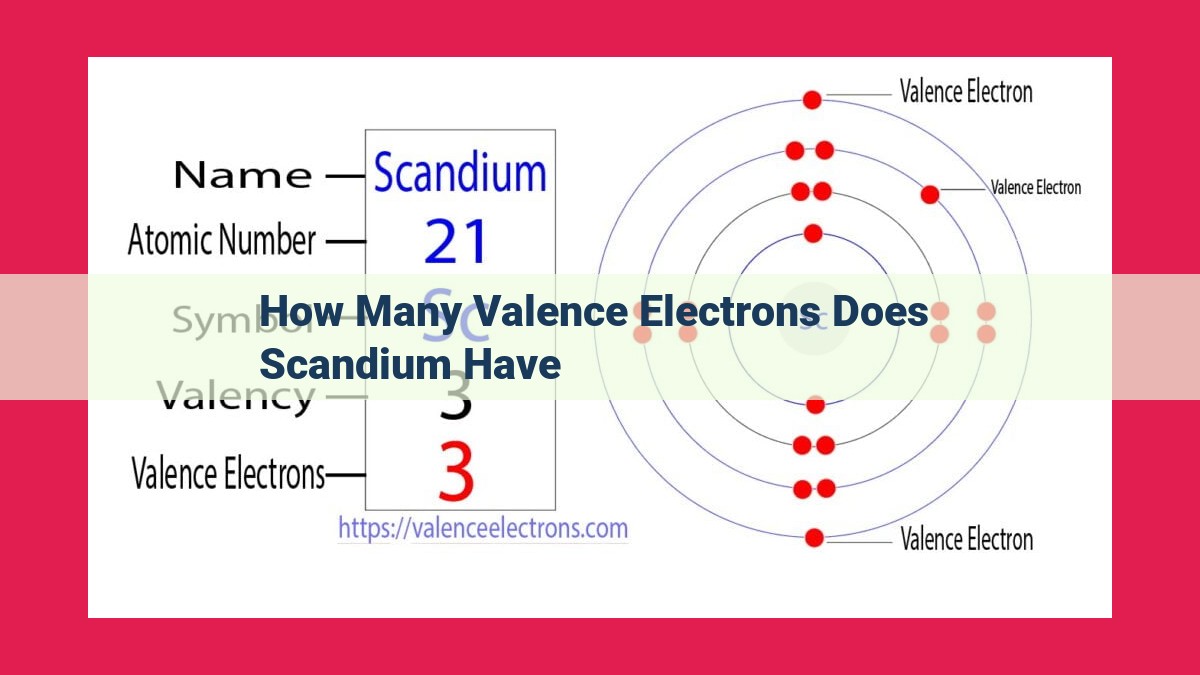
Scandium, an element in the periodic table with atomic number 21, possesses a unique electron configuration of [Ar]3d^14s^2. This configuration unveils the presence of 2 valence electrons in scandium’s outermost shell, playing a crucial role in determining its chemical properties. The number of valence electrons directly influences an element’s reactivity, making it a fundamental factor in predicting the behavior and interactions of scandium in various chemical reactions.
Understanding Valence Electrons: The Key to an Atom’s Chemical Character
In the realm of chemistry, understanding the behavior of atoms is crucial. Enter valence electrons, the pivotal players that define an atom’s chemical personality. Like tiny messengers, valence electrons dictate how an atom interacts with its surroundings, shaping its chemical prowess.
Valence electrons reside in the outermost shell of an atom, the last energy level occupied by electrons. These electrons are like the social butterflies of the atomic world, eager to mingle and form chemical bonds with neighboring atoms. The number of valence electrons an atom possesses is a significant factor in determining its chemical properties.
A Chemical Dance: The Tale of Valence Electrons
Imagine a group of dancers performing a graceful waltz. The dancers represent valence electrons, their graceful movements symbolizing the formation of chemical bonds. Atoms with a higher number of valence electrons tend to be more reactive, like eager dancers seeking partners. Conversely, atoms with fewer valence electrons are less reactive, like aloof dancers who prefer to keep their distance.
This dance of valence electrons is crucial for understanding chemical reactions. When atoms with complementary numbers of valence electrons waltz together, they form stable chemical bonds. These bonds lead to the creation of molecules, the building blocks of matter.
Electron Configuration: A Blueprint of Atomic Structure
To understand valence electrons, we must delve into the concept of electron configuration. Electron configuration describes the arrangement of electrons within an atom’s energy levels, providing a blueprint of its atomic structure. It reveals the number of electrons in each shell, including the valence electrons in the outermost shell.
Electron configuration is closely related to an element’s atomic number, which represents the number of protons in its nucleus. By understanding an element’s atomic number and electron configuration, we can predict its chemical properties and behavior.
Electron Configuration: A Map of Atomic Structure
Atoms, the fundamental building blocks of matter, are intricate entities with a story to tell. Unveiling the secrets of atomic structure is akin to embarking on an exciting journey, where electron configuration serves as a guide.
Picture electrons as miniature celestial bodies orbiting the atom’s nucleus like planets around a star. Electron configuration is a blueprint that describes the arrangement of these electrons in distinct energy levels or shells. Each shell has a specific capacity, starting with a maximum of two electrons in the first shell, increasing to eight electrons in the second and third shells.
The atomic number of an element, an unchangeable characteristic, plays a crucial role in shaping electron configuration. The atomic number dictates the number of protons found in the nucleus, which in turn determines the number of electrons the atom possesses. As we traverse the periodic table, the atomic number increments, adding one proton and one electron with each step.
For instance, consider hydrogen, the first element with an atomic number of 1. Its electron configuration is simply 1s^1, indicating a solitary electron in its first shell. Conversely, carbon, with an atomic number of 6, has an electron configuration of 1s^2 2s^2 2p^2. This notation signifies that carbon’s electrons reside in two different shells: two electrons in the first shell (1s^2) and four electrons in the second shell (2s^2 2p^2).
Understanding electron configuration is akin to reading a celestial map, revealing the distribution of electrons throughout an atom’s energy levels. By deciphering this map, we gain insight into an element’s chemical properties and behavior, laying the foundation for comprehending the intricate tapestry of molecular interactions.
The Valence Shell: Home to Reactive Electrons
- Define the valence shell as the outermost electron shell.
- Emphasize the significance of the number of valence electrons in determining reactivity.
The Valence Shell: Home to Reactive Electrons
Imagine an atom as a miniature solar system, with positively charged protons at the core, representing the sun, and negatively charged electrons orbiting around them, like tiny planets. The outermost orbit of the solar system is crucial for understanding an atom’s chemical behavior, as it holds the valence electrons.
These valence electrons are the most distant from the positively charged protons and are the most easily removed or shared during chemical reactions. The number of valence electrons an atom possesses plays a key role in determining its reactivity, as it influences the atom’s ability to form bonds with other atoms.
The valence shell is the outermost shell of electrons orbiting the atom’s nucleus. It’s like the outermost ring of a solar system, where valence electrons reside. Atoms with a large number of valence electrons are highly reactive because these electrons are loosely held and can be easily lost or transferred to form bonds with atoms of other elements.
For instance, an atom with two valence electrons, like beryllium, is more reactive than an atom with one valence electron, like sodium. This difference in reactivity arises from the number of valence electrons available for bonding. The more valence electrons an atom has, the greater its tendency to share and form chemical bonds.
Understanding the concept of valence electrons is essential for comprehending chemical reactivity and predicting how elements interact with each other. The valence shell acts as a gateway to an atom’s chemical personality, determining its ability to form bonds, share electrons, and participate in chemical reactions.
Scandium: Unveiling the Secrets of Valence Electrons
Understanding Valence Electrons and Their Role in Scandium’s Chemical Behavior
In the tapestry of the periodic table, scandium stands as a fascinating metallic element. Its atomic number, 21, reveals its unique electronic structure, which holds the key to understanding its chemical behavior. The concept of valence electrons, the electrons residing in the outermost shell of an atom, plays a pivotal role in shaping this element’s reactivity.
Scandium’s Electronic Configuration: A Blueprint of Its Atomic Structure
The electron configuration of scandium, [Ar]3d^14s^2, provides a roadmap to its atomic structure. The 18 electrons in its inner shell, denoted by [Ar], form a stable core. The remaining 3 electrons occupy higher energy levels, with two residing in the outermost shell, the valence shell.
Significance of Valence Electrons: A Window into Scandium’s Reactivity
The number of valence electrons profoundly influences an element’s chemical behavior. In the case of scandium, its two valence electrons endow it with a moderate reactivity. This means that scandium readily forms chemical bonds, particularly with non-metallic elements, to achieve a stable electron configuration.
Unveiling Scandium’s Valence Electrons: Revealing Its Chemical Potential
Scandium’s electron configuration clearly indicates the presence of two valence electrons. These electrons, located in the outermost shell, are the primary participants in chemical reactions. They determine the element’s ability to donate or accept electrons, forming bonds with other atoms.
In conclusion, valence electrons are central to understanding the chemical behavior of scandium. Its two valence electrons, nestled in the outermost shell, dictate its moderate reactivity and its propensity to form chemical bonds. By unraveling the secrets of valence electrons, scientists gain valuable insights into the fundamental properties of this intriguing element.
Unveiling Scandium’s Valence Electrons: A Tale of Reactivity
Let’s embark on a journey to unravel the secrets of scandium’s valence electrons. Valence electrons, the electrons residing in the outermost energy level, play a crucial role in determining an element’s chemical nature. And for scandium, this understanding holds the key to its unique behavior.
Electron configuration, a blueprint of an atom’s electron distribution, provides the roadmap to identifying valence electrons. Scandium, with an atomic number of 21, boasts an electron configuration of [Ar]3d^14s^2. This configuration tells us that scandium’s 18 core electrons occupy the inner shells, while the remaining two electrons reside in the outermost 4s shell.
These two valence electrons become the driving force behind scandium’s reactivity. They are like eager participants in the chemical dance, eager to interact with other atoms. The presence of just two valence electrons gives scandium a low ionization energy, making it prone to losing these electrons and forming positive ions. This characteristic explains scandium’s tendency to form stable compounds with elements that have a high electronegativity, such as oxygen and halogens.
Understanding the significance of valence electrons in scandium’s chemistry allows us to predict its behavior and its potential in various chemical reactions. It’s like having a secret weapon in our arsenal of knowledge, unlocking the door to unraveling the mysteries of the atomic world.