Salt Bridges In Electrochemical Cells: Ensuring Electrical Neutrality And Ion Exchange
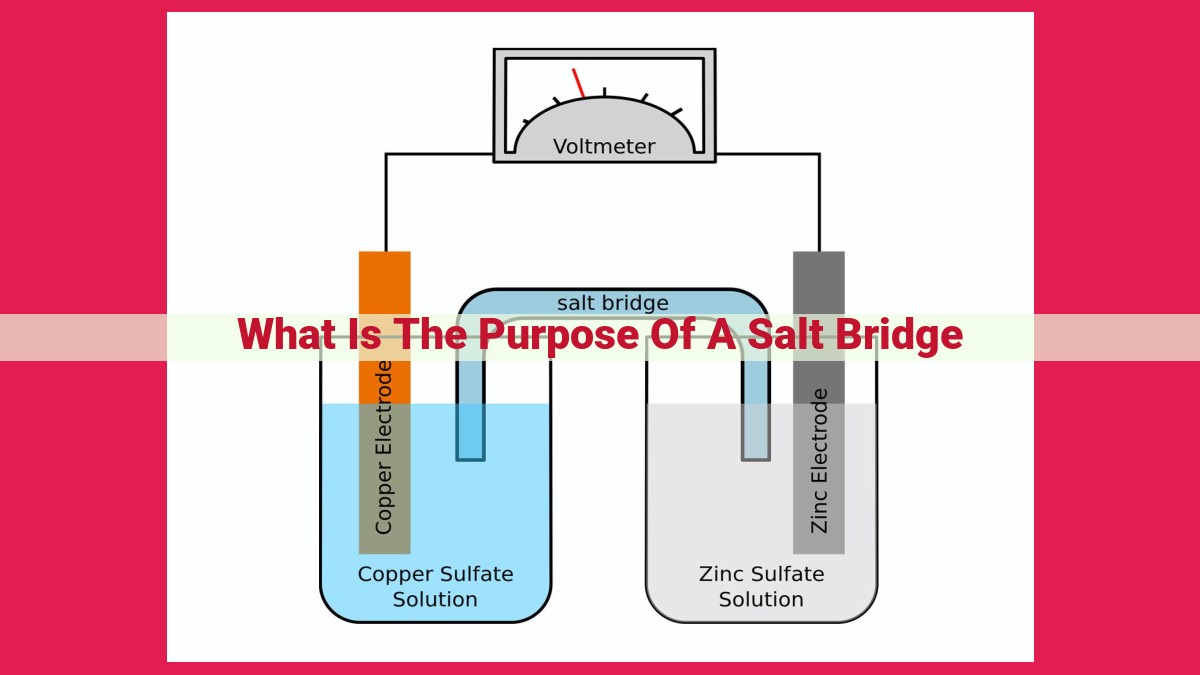
A salt bridge in an electrochemical cell maintains electrical neutrality by providing a conductive link between half-cells. It allows ion exchange, which enables current flow and prevents charge imbalances during electrochemical reactions. The salt bridge facilitates ion migration, driven by electrical potential difference, and helps maintain electrical conductivity while separating charge. Its presence completes the electrical circuit, allowing electron flow and facilitating various electrochemical processes.
Salt Bridges: The Unsung Heroes of Electrochemical Cells
Electrochemical cells are like tiny powerhouses, converting chemical energy into electrical energy. They play a vital role in batteries, fuel cells, and other devices that shape our modern world. But behind the scenes, these cells have a hidden ally—the salt bridge.
Electrochemical Cells: The Powerhouses of Energy Conversion
Imagine an electrochemical cell as a divided kingdom, with two warring factions (anode and cathode) separated by a border (electrolyte solution). The anode and cathode are like opposing electrical poles, eager to exchange electrons to create a flow of electricity.
Electrolyte Solutions: The Conductive Bridge
The electrolyte solution, filled with charged ions, serves as the communicative bridge between these warring factions. Ions, with their electric charge, can roam freely through the solution, carrying the electrical current.
The Salt Bridge: The Peacekeeper
Now, enter the salt bridge, a noble intermediary connecting the two half-cells. Its presence ensures that the electrical neutrality is maintained even as ions migrate from one side to the other, preventing chaos within the cell.
A Circuit for Charge Flow
The salt bridge completes the electrical circuit, allowing electrons to flow from anode to cathode. This flow of electrons creates an electrical current, delivering the power we need to operate our devices.
Ion Migration: The Path to Separation
Like a skilled diplomat, the salt bridge facilitates the movement of ions between the half-cells, ensuring that charge is separated and imbalance is avoided. This ion migration is driven by the electrical potential difference between the anode and cathode.
Electrical Potential Difference: The Guiding Force
The electrical potential difference, like a commanding general, dictates the direction of ion movement. It creates an electric field that propels ions toward the oppositely charged electrode, maintaining electrical neutrality throughout the cell.
The salt bridge, though often overlooked, is an indispensable component of electrochemical cells. It ensures electrical conductivity, maintains charge balance, and keeps the cell operating smoothly. Without this humble yet essential player, our electrochemical devices would falter, leaving us devoid of the conveniences that power our modern lives.
The Vital Role of Salt Bridges in Electrochemical Cells
Electrolyte Solutions: The Conductive Foundation
Imagine an electrochemical cell as a bustling city, where electricity flows like traffic through a network of electrolyte solutions. These solutions are like busy streets, teeming with ions, the charged particles that make electrical conductivity possible. These ions, like tiny cars, carry electrical charges throughout the cell, enabling the smooth flow of electricity.
Electrolyte solutions are composed of ionic compounds, such as sodium chloride (NaCl), which dissociate in water, forming separate positive and negative ions. These ions are like magnets, attracted to opposite charges on the electrodes of the electrochemical cell. Just as cars follow designated lanes, ions move through electrolyte solutions, creating a continuous path for electrical current.
The Salt Bridge: A Conductive Link
In the heart of the electrochemical cell, a salt bridge acts as a vital connecting point between the two half-cells, each containing one electrode. The salt bridge is a tube filled with a concentrated electrolyte solution that allows ions to flow freely. These ions serve as a bridge, connecting the two halves of the cell and maintaining electrical neutrality throughout the system.
Complete Circuit: The Pathway for Electron Flow
Electrochemical cells function based on the principles of electrical circuits, where current flows through a complete path. In an incomplete circuit, there is no continuous path, preventing electron flow. However, in a complete circuit, the salt bridge provides the missing link, creating a pathway for ions to move and electrons to flow.
Ion Migration: The Driving Force
The movement of ions through the salt bridge is driven by an electrical potential difference between the electrodes of the cell. This potential difference acts like a magnet, attracting positively charged ions to the negative electrode (cathode) and negatively charged ions to the positive electrode (anode).
Electrical Potential Difference: The Guiding Light
The electrical potential difference between the electrodes is carefully controlled and maintained by the salt bridge. This difference drives the migration of ions and ensures that the cell remains electrically neutral. The salt bridge balances the potential difference between the half-cells, preventing excessive buildup of charge on either side.
The salt bridge is an indispensable component of electrochemical cells. It provides a conductive link between the half-cells, allowing ions to flow freely and maintaining electrical neutrality. Without the salt bridge, the cell would be an incomplete circuit, unable to generate an electrical current.
Highlight their role in transferring ions, enabling current flow in electrochemical cells.
Electrochemical Cells: The Vital Role of Salt Bridges
In the realm of electrochemistry, electrochemical cells are like tiny powerhouses that convert chemical energy into electrical energy. And at the heart of these cells lies a crucial component: the salt bridge.
Picture this: an electrochemical cell is like a playground, split into two separate sections called half-cells. Each half-cell has its own unique chemical reaction going on, creating a difference in electrical potential between them. This difference is like a spark, a driving force that sets the stage for an electrical current to flow.
But here’s the catch: these half-cells can’t directly connect. They need a neutral bridge, a mediator to allow ions to flow and complete the circuit. Enter the salt bridge.
The Conductive Lifeline
A salt bridge is a simple yet ingenious solution. It’s a U-shaped tube filled with an electrolyte solution, a liquid packed with electrically charged particles called ions. These ions are the key to unlocking the flow of electricity.
When the salt bridge is submerged in the two half-cells, it acts as a gateway, allowing ions to travel from one side to the other. This movement of positive ions (cations) and negative ions (anions) creates an ion migration that completes the circuit.
Maintaining Balance
The salt bridge plays another crucial role: it maintains electrical neutrality. As ions flow from one half-cell to the other, the salt bridge ensures that an equal number of ions are exchanged, preserving the overall charge balance of the system. This delicate equilibrium ensures that the reaction in the electrochemical cell can continue smoothly.
The Driving Force
The driving force behind ion migration is the electrical potential difference between the half-cells. This difference is like a voltage difference, a push that compels ions to move. The salt bridge balances this potential difference, allowing ions to flow in a controlled manner.
The Importance of Completeness
Without a salt bridge, the electrochemical cell would be incomplete, like a puzzle with missing pieces. The ions would not be able to complete their journey, and the electrical current would not be able to flow. The salt bridge, therefore, completes the circuit, enabling the cell to function as intended.
In the world of electrochemical cells, the salt bridge is an unsung hero, a vital component that ensures the smooth flow of ions and the maintenance of electrical neutrality. Its presence enables electrochemical cells to harness chemical energy and produce the electricity that powers our devices and illuminates our world.
Electrochemical Cells: Unlocking the Power of Salt Bridges
Electrochemical cells harness the transformative power of chemical energy to generate electrical energy, a process that underlies countless technologies powering our modern world. At the heart of these cells lies a seemingly simple yet crucial component: the salt bridge.
An electrochemical cell comprises two half-cells, each consisting of an electrode immersed in an electrolyte solution. These half-cells are separated by a porous barrier, known as the salt bridge. Its primary function is to connect the two half-cells, allowing ions to flow freely while preventing the mixing of the electrolyte solutions.
Imagine the salt bridge as a conductive pathway, a lifeline between the half-cells. This pathway enables the migration of ions from one half-cell to the other, a process essential for charge separation and the generation of electrical current.
Explain how the salt bridge maintains electrical neutrality by allowing ion exchange.
The Salt Bridge: A Conductive Conduit in Electrochemical Cells
In the realm of electrochemical cells, the quest for electrical conductivity and charge balance is paramount. Among the cast of characters that ensure this delicate dance, the salt bridge stands out as an essential orchestrator.
Visualize an electrochemical cell, a playground where chemical reactions spark a flow of electrons. Two half-cells, each teeming with ions, face off, separated by a porous salt bridge. Like a permeable membrane, this bridge allows ions to traverse its depths, maintaining the electrical neutrality so crucial to the cell’s operation.
As the electrons waltz from one electrode to another, a dance of ion migration ensues. From the anode’s hungry embrace, positive ions embark on a journey, eager to fill the void left by departing electrons. They surge towards the bridge, seeking a path to the cathode. Simultaneously, negative ions abandon the cathode’s embrace, drawn by the electrical potential difference towards the anode.
The salt bridge serves as their conduit, a neutral haven where ions exchange places, ensuring that the overall _electrical charge remains balanced. The bridge prevents a charge buildup on either side, allowing the current to flow seamlessly.
In this dance of ions, the electrical potential difference acts as the conductor. It drives the ions, like marionettes, towards their appointed destinations. The salt bridge, with its uncanny ability to facilitate ion exchange, ensures that the electrical neutrality required for this harmonious flow is maintained.
Without this conductive link, the electrochemical cell would falter, unable to sustain the electron flow that drives its reactions. The salt bridge stands as an unsung hero, its role pivotal in maintaining the delicate balance that empowers electrochemical cells.
Understanding the Heart of Electrochemical Cells: The Role of Salt Bridges
Electrochemical cells are fascinating devices that harness chemical energy to generate electricity. At the heart of these cells lies a critical component known as a salt bridge, which plays an essential role in maintaining electrical flow and charge balance.
Electrical Current, Voltage, and Resistance: The Guiding Forces
To comprehend the significance of salt bridges, we must delve into the concepts of electrical current, voltage, and resistance. Electrical current, measured in amperes (A), represents the flow of electrically charged particles (ions). Voltage (V), on the other hand, is the electrical potential difference between two points in a circuit, driving the movement of ions. Resistance (Ω), often compared to friction, opposes the flow of current, affecting the strength of the electrical signal.
The Incomplete Circuit and the Salt Bridge’s Intervention
Imagine an electrochemical cell as a circuit. When the cell is disconnected, the circuit remains incomplete, preventing the flow of electrical current. The salt bridge serves as the missing link, completing the circuit and allowing the passage of ions. It acts as a conduit, providing a pathway for ions to migrate between the two half-cells of the electrochemical cell.
The Electrical Potential Difference and the Salt Bridge’s Balancing Act
The salt bridge plays a pivotal role in balancing the electrical potential difference between the two half-cells, creating a driving force for ion migration. This difference in potential drives the ions from the anode (positive electrode) towards the cathode (negative electrode). The salt bridge facilitates this ion movement, ensuring that the cell remains electrically balanced.
The Salt Bridge’s Importance: A Summary
In summation, the salt bridge in an electrochemical cell performs several crucial tasks:
- Completes the circuit: Provides a pathway for ion flow, enabling electrical current to flow.
- Facilitates ion migration: Enables the movement of ions from anode to cathode, separating charge.
- Balances the electrical potential difference: Maintains equilibrium between the half-cells, ensuring charge neutrality.
In essence, salt bridges are the unsung heroes of electrochemical cells, orchestrating the smooth and efficient flow of ions, electricity, and charge. Without them, the generation of electricity from chemical reactions would be impossible.
The Salt Bridge: A Conductive Link in Electrochemical Cells
When you picture an electrochemical cell, you might envision a simple setup with two electrodes submerged in a liquid. But there’s an often-overlooked component that plays a crucial role in making these cells work: the salt bridge.
Let’s imagine an electrochemical cell as two half-cells, each containing an electrode immersed in an electrolyte solution. As ions flow between the electrodes, an electrical potential difference arises between them. To prevent the buildup of charge imbalance in the cell, a complete electrical circuit is necessary.
This is where the salt bridge steps in. It connects the two half-cells, providing a pathway for ions to flow and maintain charge balance. The salt bridge contains a concentrated solution of an inert electrolyte, such as potassium chloride (KCl).
In an incomplete circuit, ions can’t move between the half-cells, leading to charge buildup and the cell becoming non-functional. But with a salt bridge present, ions are free to travel from the anode (where oxidation occurs) to the cathode (where reduction occurs), completing the circuit.
This ion movement creates a balance of electrical potential between the half-cells. As ions migrate, the separated charges at the electrodes gradually neutralize, allowing the electrochemical cell to function continuously.
Discuss the electrical potential difference between electrodes and its impact on ion migration.
The Electrical Potential Difference: A Driving Force for Ion Migration
The electrical potential difference between the electrodes in an electrochemical cell plays a crucial role in driving the movement of ions across the salt bridge. This potential difference arises from the difference in electrical charge between the two electrodes, with the anode being positively charged and the cathode being negatively charged.
As a result of this potential difference, ions in the electrolyte solution are attracted to the oppositely charged electrodes. Positive ions (cations) migrate towards the cathode, while negative ions (anions) migrate towards the anode. This movement of ions creates an ion current that flows through the salt bridge, completing the electrical circuit.
The salt bridge acts as a conductive pathway for this ion current, allowing ions to move freely between the two half-cells without significantly altering the overall charge balance of the system. This ion migration is essential for maintaining the electrical potential difference between the electrodes and, ultimately, for the functioning of the electrochemical cell.
Ion Migration: The Driving Force
Imagine a bustling city with two power plants, each generating electricity. For the city to function, electricity must flow from the power plants to homes and businesses. However, a missing link prevents the current from completing its journey—a gap exists between the power plants.
In an electrochemical cell, similar to our city, two half-cells act as power plants, generating electrical potential. But just like the missing link in the city’s power grid, a gap exists between the half-cells. This gap prevents the flow of electrical current until a salt bridge steps in.
The salt bridge acts as a conductive pathway, bridging the gap between the half-cells. It contains electrolyte solution, a special liquid filled with ions (charged particles), creating a path for ions to move freely.
Like eager travelers, positive ions embark on a journey from the anode (negative electrode) to the cathode (positive electrode). Meanwhile, negative ions embark on the opposite adventure, from the cathode to the anode. This movement of ions separates charge, creating an electrical potential difference between the half-cells, driving the flow of electricity.
The salt bridge ensures that this ion exchange occurs smoothly, maintaining electrical neutrality in the entire system. Without the salt bridge, the circuit would remain incomplete, and the electrochemical cell would be unable to generate a sustainable electrical current. The salt bridge, therefore, plays a crucial role in facilitating the movement of ions, separating charge, and enabling the flow of electricity in electrochemical cells.
The Unsung Hero of Electrochemical Cells: Salt Bridges
The Essence of Salt Bridges
Electrochemical cells are like the powerhouses of our devices, enabling the flow of electrons that charge our batteries and power our lives. At the heart of these cells lies a seemingly simple yet crucial component: the salt bridge.
Electrolyte Solutions: The Conductive Medium
Electrochemical cells rely on electrolyte solutions, liquids teeming with charged particles called ions. These ions, like tiny messengers, carry electrical charge and allow the flow of electricity.
The Salt Bridge: A Conductive Link
The salt bridge, a porous bridge connecting the two half-cells of an electrochemical cell, is made of a saturated solution of an inert electrolyte, such as sodium chloride (NaCl). Its porous structure allows ions to move freely between the half-cells.
Complete Circuit: The Pathway for Electrons
Electrical current flows when there is a complete circuit, a closed loop where electrons can travel. The salt bridge completes this circuit by providing a path for ions to exchange, maintaining electrical neutrality in both half-cells.
Ion Migration: The Driving Force
The electrical potential difference between the electrodes in electrochemical cells creates an electrical gradient. This gradient drives the movement of ions from the anode (negative electrode) to the cathode (positive electrode), separating charge and creating an electrical current.
Electrical Potential Difference: The Guiding Light
The electrical potential difference, measured in volts, quantifies the driving force for ion migration. The salt bridge helps balance the potential difference between the half-cells, ensuring a continuous flow of ions.
The salt bridge, though often overlooked, plays a pivotal role in electrochemical cells. By providing a conductive link between the half-cells, it facilitates the flow of ions and maintains electrical neutrality, enabling the efficient flow of electrical current. Without the unsung hero of salt bridges, electrochemical cells would simply not function.
Salt Bridges: The Conductive Lifeline of Electrochemical Cells
Electrochemical cells are intricate systems that spark chemical reactions through electrical energy. A crucial component of these cells, often overlooked, is the salt bridge, a vital conductive link that ensures their proper functioning.
Electrolyte Solutions: The Ionic Highway
Within electrochemical cells resides an electrolyte solution—a liquid brimming with ions, the charged messengers of electricity. These ionic solutions serve as the highway upon which ions travel, enabling the flow of electricity.
The Salt Bridge: A Vital Connection
The salt bridge is an ingenious connection between the two half-cells that make up an electrochemical cell. It bridges the gap between them, allowing ions to migrate, a crucial process that ensures the cell’s functionality.
Maintaining Electrical Neutrality: The Balancing Act
The salt bridge plays a pivotal role in maintaining electrical neutrality within the cell. As ions migrate from one half-cell to another, the salt bridge simultaneously allows oppositely charged ions to flow in the opposite direction, preventing an imbalance of charge.
Complete Circuit: The Pathway to Electron Flow
The salt bridge completes the circuit within the electrochemical cell, enabling the flow of electrons. This flow generates an electrical current that triggers the desired chemical reactions. Without the salt bridge, the circuit would remain incomplete, and no reaction would occur.
Electrical Potential Difference: The Driving Force
An electrical potential difference exists between the electrodes of an electrochemical cell. This difference drives the migration of ions across the salt bridge. As ions move from the anode to the cathode, they separate charge, creating an electrical potential difference that maintains the flow of current.
The Salt Bridge: A Balancing Force
The salt bridge plays a critical role in balancing the electrical potential difference between the half-cells. It ensures that the potential difference remains stable, allowing a continuous flow of ions and maintaining the electrochemical equilibrium necessary for the cell to function.
Electrochemical Cells: The Salt Bridge’s Pivotal Role
In the realm of electrochemistry, electrochemical cells play a central role in converting chemical energy into electrical energy. But what truly makes these cells tick? Enter the salt bridge, an unassuming yet indispensable component responsible for maintaining the delicate balance within these electrochemical setups.
Imagine two half-cells, each bustling with activity. On one side, the anode, electrons dance away from the metal, leaving behind positively charged ions. On the other, the cathode, electrons eagerly await their arrival. But how do these electrons navigate the divide? That’s where the salt bridge steps in.
Like a porous bridge spanning the gap between half-cells, the salt bridge allows ions to flow freely. Negatively charged ions from the electrolyte solution surrounding the anode migrate towards the cathode, while positively charged ions head in the opposite direction. This ionic migration creates a complete circuit, allowing electrons to flow from anode to cathode, producing an electrical current.
The salt bridge, far from being a mere conduit for ions, plays a crucial role in maintaining electrical neutrality. As electrons leave the anode, positively charged ions accumulate, while at the cathode, the influx of electrons leads to a surplus of negative charge. The salt bridge, by facilitating the movement of ions, prevents this charge imbalance, ensuring the smooth flow of electrons and the continued generation of electrical energy.
In essence, the salt bridge acts as a mediator, harmonizing the chemical reactions within electrochemical cells. Its presence ensures the proper flow of ions, which in turn sustains electrical current and maintains electrical neutrality. Without the salt bridge, electrochemical cells would falter, unable to fulfill their role in harnessing the power of chemical reactions to produce electricity.
Conclude with the importance of salt bridges in enabling electrical conductivity and maintaining charge balance.
Salt Bridges: The Unsung Heroes of Electrochemical Cells
In the world of electricity, electrochemical cells are the powerhouses that convert chemical energy into electrical energy. At the heart of these cells lies a humble yet indispensable component called a salt bridge.
Imagine an electrochemical cell as two separate compartments connected by a bridge. Inside these compartments, special solutions called electrolyte solutions contain ions, which are electrically charged particles. To create an electrical circuit, these ions need to flow from one compartment to the other.
This is where the salt bridge comes into play. It’s a simple, U-shaped tube filled with another electrolyte solution. It serves as a conductive link between the two compartments.
As ions accumulate in one compartment, the salt bridge provides a pathway for them to travel to the other compartment. This ion exchange helps maintain electrical neutrality throughout the cell. Ions from the anode (negative electrode) migrate to the cathode (positive electrode), and vice versa.
By facilitating this ion migration, the salt bridge creates a complete circuit for electron flow. Electrons flow from the anode to the cathode outside the cell, while ions flow within the cell through the salt bridge. This creates an electrical current, which is the foundation of electrical energy.
The salt bridge also plays a crucial role in maintaining an electrical potential difference between the compartments. This potential difference drives the ion migration, ensuring a continuous flow of electrons and current.
In conclusion, the salt bridge is an unassuming but essential component of electrochemical cells. It provides a conductive path for ion exchange, which enables electrical conductivity and maintains charge balance. Without the salt bridge, the cell would be incomplete, and the flow of electricity would be impossible.