Salt Bridges In Electrochemical Cells: Essential Components For Accurate Measurements And Redox Reactions
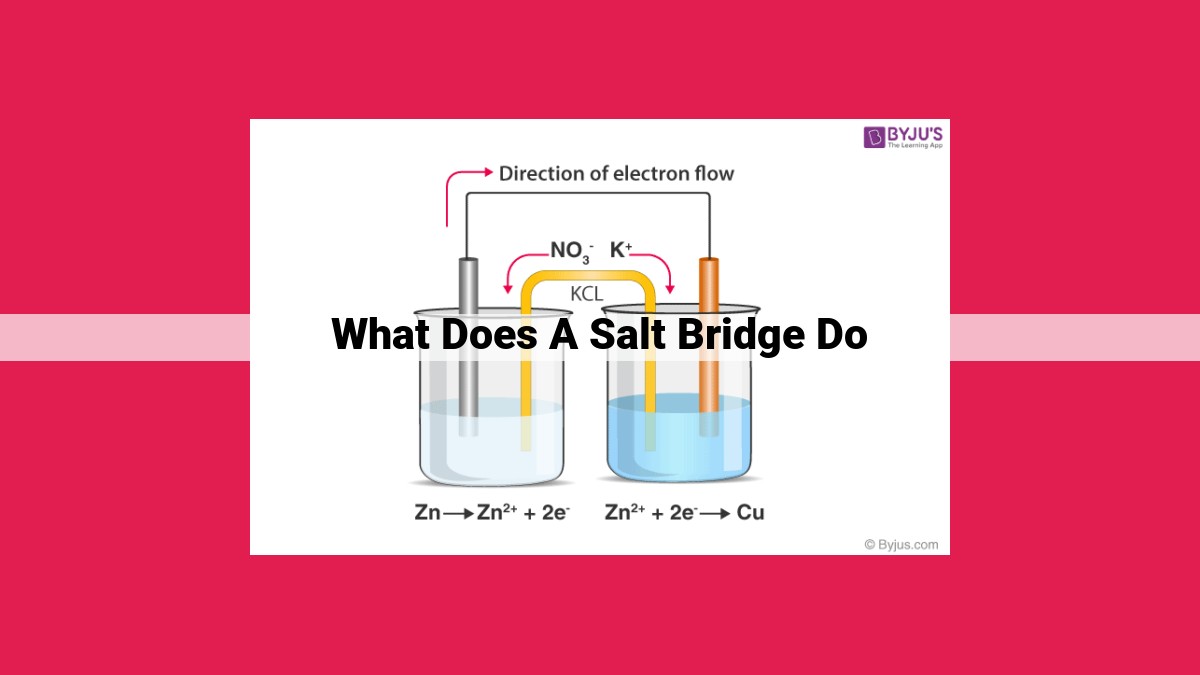
A salt bridge in an electrochemical cell serves multiple functions: maintaining electrical neutrality by preventing charge accumulation, facilitating ion migration between half-cells to complete the circuit, preserving constant ionic strength, ensuring solutions remain separated, and minimizing liquid junction potential interference. By maintaining equilibrium and ion flow, salt bridges enable redox reactions and accurate electrochemical measurements.
The Intricate Role of Salt Bridges in Maintaining Electrochemical Equilibrium
In the realm of electrochemistry, salt bridges play an indispensable role in ensuring the seamless functioning of electrochemical cells. These ingenious devices are responsible for maintaining electrical neutrality within the cell, allowing the intricate dance of redox reactions to unfold.
Neutralizing Charge Buildup
Electrochemical cells are essentially the battleground of positively and negatively charged ions. As ions flow through the cell, they create pockets of charge buildup that could disrupt the delicate balance of the system. Enter the salt bridge, a porous barrier filled with an electrolyte solution. This solution allows ions to pass through it, effectively neutralizing any charge buildup.
This constant exchange of ions across the salt bridge ensures that both half-cells of the electrochemical cell maintain an equal and opposite charge. Without this charge balance, the electrochemical reactions would cease, and the cell would become inert.
Function 2: Facilitating Ion Migration in Electrochemical Cells
In the realm of electrochemistry, the dance of ions takes center stage, orchestrating the exchange of electrical energy and chemical change. Salt bridges play a pivotal role in this ionic ballet, enabling the smooth flow of ions between half-cells, completing the electrical circuit, and nourishing redox reactions.
Picture this: Two half-cells, each an electrochemical playground, separated by a porous barrier. Within these chambers, ions eagerly await the signal to move, their potential energy itching to be unleashed. As a reaction unfolds in one half-cell, hungry electrons seek partners in the other. But alas, a physical divide stands between them.
Enter the salt bridge, a cleverly crafted pathway that bridges this divide, allowing ions to embark on their journey of migration. Like microscopic couriers, ions traverse the salt bridge, carrying their electrical charge and fostering the exchange of electrons.
This ion migration not only completes the electrical circuit but also fuels redox reactions, the heartbeats of electrochemical cells. Redox reactions, where electrons are enthusiastically exchanged, rely on the constant movement of ions between half-cells. The salt bridge, by ensuring the seamless flow of ionic messengers, keeps the redox dance alive.
Without the salt bridge, electrochemical cells would be silenced, their potential stifled by the buildup of charge and the hindered migration of ions. It is the salt bridge that orchestrates the ionic symphony, enabling these cells to harness the power of redox reactions and perform their remarkable electrochemical transformations.
Function 3: Maintaining Constant Ionic Strength
In electrochemistry, ionic strength refers to the concentration of ions in a solution. Maintaining a constant ionic strength is crucial for accurate measurements and efficient electrochemical reactions. Salt bridges play a vital role in preserving this delicate balance.
Imagine an electrochemical cell without a salt bridge. As ions flow between the half-cells, the ionic strength in one half-cell may increase while it decreases in the other. This imbalance creates a concentration gradient, leading to unwanted reactions and interfering with the primary electrochemical process.
Salt bridges act as a stabilizing force, preventing these fluctuations in ionic strength. By providing a continuous path for ion migration, salt bridges allow ions to move freely between the half-cells, distributing them evenly. This uniform distribution ensures that the ionic strength remains constant throughout the cell, optimizing the electrochemical reactions and producing more reliable results.
**Preventing Unwanted Mixing: The Crucial Role of Salt Bridges in Electrochemical Cells**
In the intricate world of electrochemical cells, salt bridges play a pivotal role in maintaining pristine isolation between distinct solutions. Their presence physically separates these solutions, ensuring that unwanted mixing does not disrupt the delicate balance of electrochemical reactions.
Imagine two isolated solutions, each containing a different set of ions. Without a salt bridge, these solutions would eagerly interact, their ions intermingling and creating a chaotic blend. However, the introduction of a salt bridge creates a controlled pathway for ion movement, allowing them to migrate selectively between the two compartments.
A salt bridge typically consists of a porous material saturated with a highly concentrated salt solution. This concentrated solution acts as a reservoir of ions, providing a steady supply to maintain the ionic balance in both compartments. As ions are drawn from the salt bridge to balance charge buildup, an equal number of ions from the compartments replenish the salt bridge, preserving their ionic concentrations.
This controlled separation ensures that the chemical reactions occurring in each compartment remain in isolation. The reactants and products are confined to their respective environments, preventing cross-contamination and allowing the desired electrochemical reactions to proceed unhindered.
Thus, salt bridges serve as guardians of electrochemical purity, preventing unwanted solution mixing and ensuring that reactions occur under controlled conditions. Their presence is essential for accurate electrochemical measurements and the reliable operation of electrochemical cells.
Function 5: Minimizing Liquid Junction Potential
When two solutions with different ionic concentrations meet, a boundary called a liquid junction is formed. At this junction, ions tend to diffuse from one solution to the other, creating a potential difference. This phenomenon is known as liquid junction potential.
In electrochemical cells, liquid junction potential can interfere with accurate measurements by introducing an additional voltage that is not part of the desired electrochemical reaction. To minimize this interference, salt bridges are used.
Salt bridges are typically made of an inert material, such as agarose, which contains a concentrated solution of an electrolyte (ions in solution). These salt bridges allow ions to flow between the half-cells, completing the electrical circuit and supporting redox reactions.
By separating the solutions with a salt bridge, the liquid junction potential is minimized because the concentration gradient between the solutions is reduced. The salt bridge also prevents significant mixing of the solutions, ensuring that the reactions occur in isolation.
In summary, salt bridges in electrochemical cells play a crucial role in maintaining electrical neutrality, facilitating ion migration, preserving constant ionic strength, preventing solution mixing, and minimizing liquid junction potential. These functions ensure accurate and reliable electrochemical measurements.