Salt Bridge: Essential Component In Electrochemical Cells For Ion Flow And Electrical Neutrality
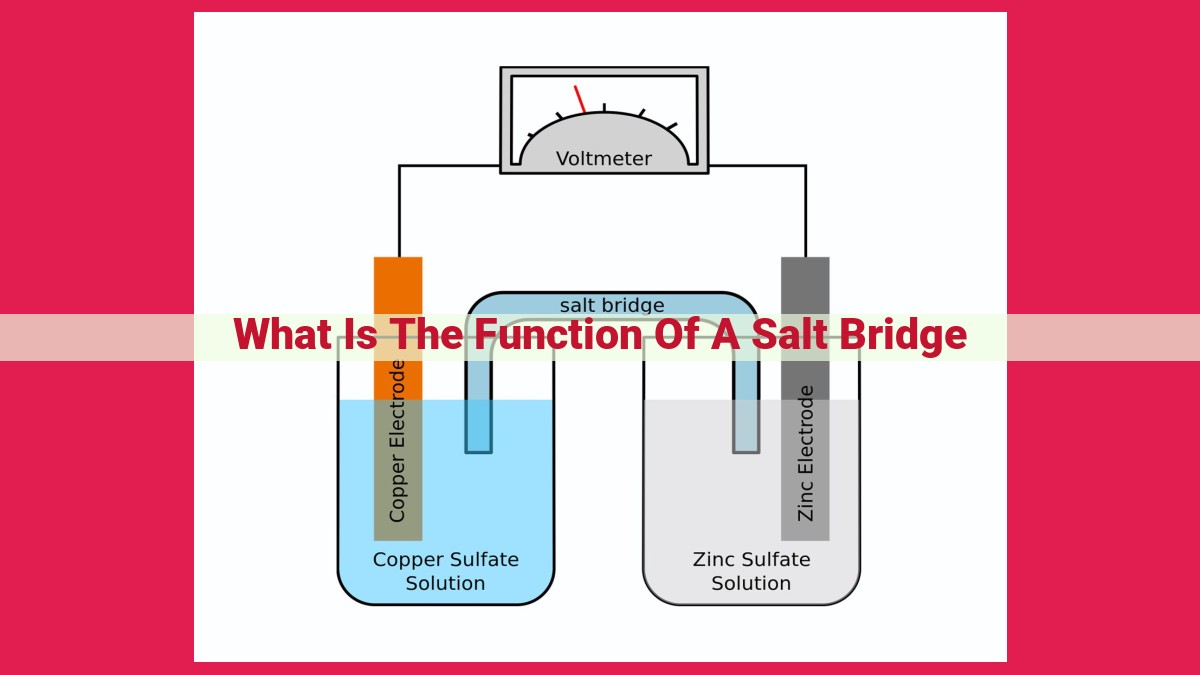
A salt bridge is a component in electrochemical cells that connects two electrolytic solutions. It allows ions to flow between the solutions, maintaining electrical neutrality and facilitating electrochemical reactions. The salt bridge completes the electrical circuit, enabling the flow of current and the transfer of electrons between the anode and cathode. It ensures that the overall charge balance is maintained during oxidation and reduction reactions, allowing electrochemical cells to function effectively.
The Vital Role of Salt Bridges in Electrochemical Cells
In the realm of electrochemistry, salt bridges play a crucial role in the seamless functioning of electrochemical cells. These remarkable devices harness the power of chemical reactions to generate electricity or drive other reactions. And at the heart of these electrochemical cells lies a humble yet essential component: the salt bridge.
Defining the Salt Bridge and Its Purpose
Imagine a salt bridge as a porous divider separating two electrolytic solutions within an electrochemical cell. This divider allows for the flow of ions between the solutions, which is vital for maintaining electrical neutrality and facilitating electrochemical reactions.
Electrolyte Solutions: The Ion Highway
Electrolytic solutions are special liquids or gels that contain dissolved ions. These ions, positively charged cations and negatively charged anions, can move freely within the solution. The salt bridge serves as a conductive pathway for these ions, allowing them to flow between the solutions and complete the electrical circuit.
Maintaining Electrical Neutrality: Balancing the Charge
In electrochemical cells, it’s crucial to maintain electrical neutrality, meaning that the total positive charge equals the total negative charge. The salt bridge plays a pivotal role in preserving this balance. As ions move through the bridge, they ensure that the charge remains equal in both solutions, preventing the buildup of excessive charge in either.
Anode and Cathode: The Reaction Hubs
Electrochemical cells consist of two electrodes known as the anode and the cathode. The anode is where oxidation (loss of electrons) occurs, while the cathode is where reduction (gain of electrons) takes place. The salt bridge connects these electrodes, allowing for the flow of ions between them and enabling the transfer of electrons necessary for electrochemical reactions.
Oxidation and Reduction: The Drivers of Electrochemistry
Oxidation and reduction reactions are the driving forces behind electrochemical cells. Oxidation occurs when a substance loses electrons, while reduction occurs when a substance gains electrons. The salt bridge facilitates electron transfer between the anode and the cathode, completing the circuit and allowing for the electrochemical reactions to proceed smoothly.
Electrolytic Solutions: The Heart of Electrochemical Cells
In the realm of electrochemistry, electrolytic solutions play a pivotal role, acting as the medium through which ions dance and electrical currents flow. These solutions are charged with ions, tiny particles carrying either a positive or negative charge. When dissolved in a solvent, these ions become mobile, ready to engage in the intricate world of electrochemistry.
Electrolytic solutions possess a unique ability to facilitate the flow of ions. When an electric field is applied, these ions become energized and embark on a synchronized movement, creating an ionic current. It is this current that powers the reactions at the heart of electrochemical cells, where chemical energy is transformed into electrical energy, and vice versa.
The salt bridge, a crucial component of electrochemical cells, serves as a conductive bridge between two electrolytic solutions, allowing ions to flow freely between them. This ionic exchange maintains electrical neutrality within the cell, ensuring that the overall charge remains balanced. Thus, the salt bridge plays an indispensable role in completing the electrical circuit, enabling the smooth flow of current and the occurrence of electrochemical reactions.
Flow of Ions in Electrochemical Cells
In the heart of electrochemical cells lies a delicate balance of ions, the tiny charged particles that carry the power of electricity. As these ions dance within the electrolytic solutions, a fascinating journey unfolds, governed by the fundamental principles of electrical neutrality.
Mechanisms and Direction of Ion Movement
Imagine a sea of ions, suspended within the electrolytic solution. Like tiny magnets, positive and negative ions are drawn to each other, eager to cancel out their opposite charges. This inherent attraction sets them in motion, creating a constant flow of ions.
Maintaining Electrical Neutrality
The harmony of electrochemical cells relies heavily on electrical neutrality, the state where overall charge is balanced and there is no net buildup. As ions move within the solutions, they must maintain this delicate equilibrium. Like a skilled juggler, the system ensures that the number of positive ions remains equal to the number of negative ions.
Role of the Salt Bridge
Enter the salt bridge, the unsung hero of electrochemical cells. This simple yet crucial component forms a porous barrier, connecting the two electrolytic solutions. It acts as a gateway, allowing ions to migrate between compartments while maintaining electrical neutrality.
When an electrical potential is applied across the cell, the ions respond like miniature race cars. Positive ions, eager to neutralize the negative charge at the cathode, rush towards it. Simultaneously, negative ions are drawn to the anode, ready to neutralize its positive charge.
As the ions race between compartments, the salt bridge ensures that charge balance is maintained. It allows ions to flow freely, preventing the buildup of excess charge and maintaining the smooth operation of the cell.
Electrical Neutrality in Electrochemical Cells
In the realm of electrochemistry, a dance of ions takes place, shaping the very essence of chemical reactions. At the heart of this ionic waltz lies a crucial concept: electrical neutrality. Maintaining a harmonious balance of charges is paramount for the smooth operation of electrochemical cells.
Electrochemical cells comprise two distinctive compartments, each filled with an electrolytic solution. These solutions, brimming with freely moving ions, serve as highways for electrical currents. As ions embark on their charged journeys, they could potentially disrupt the delicate equilibrium of the cell. However, a remarkable solution bridges the electrolytic solutions: the salt bridge.
The salt bridge, like a porous gatekeeper, straddles the divide between the compartments. Its primary mission is to facilitate the subtle exchange of ions, ensuring an overall charge balance in the cell. Without this ionic custodian, an imbalance of charges would arise, hindering the flow of current and disrupting the electrochemical reactions.
Think of the salt bridge as a mediator, orchestrating a harmonious flow of ions to maintain electrical neutrality. It allows positively charged cations to migrate from one compartment to the other, while negatively charged anions travel in the opposite direction. This delicate dance of ions ensures an overall neutral charge in both compartments, preventing any buildup of excess charges.
In this electrochemical ballet, the salt bridge plays a pivotal role, akin to the conductor in an orchestra. It synchronizes the movement of ions, ensuring that the electrical harmony of the cell is preserved. Without this ionic symphony, electrochemical reactions would falter, disrupting the intricate symphony of chemical transformations.
The Salt Bridge: A Vital Connection in Electrochemical Cells
Electrochemical cells are devices that convert chemical energy into electrical energy or vice versa. They play a crucial role in various technologies, including batteries, fuel cells, and electroplating. At the heart of these cells lies a salt bridge, a seemingly simple yet essential component that ensures the smooth flow of reactions.
In an electrochemical cell, two half-cells are connected by a salt bridge. Each half-cell contains an electrode submerged in an electrolytic solution. The anode is the electrode where oxidation occurs, the loss of electrons. Conversely, the cathode is where reduction takes place, the gain of electrons.
The salt bridge, like a hidden pathway, connects the anode and cathode, forming a complete electrical circuit. This connection allows ions to flow between the two half-cells, maintaining electrical neutrality and facilitating the transfer of electrons.
Electrical neutrality is the delicate balance of charges within an electrochemical cell. As positive ions (cations) move towards the cathode, negative ions (anions) migrate towards the anode through the salt bridge. This continuous ion exchange maintains an equal distribution of charges, preventing an electrical imbalance.
The flow of ions through the salt bridge is crucial for the overall functioning of the electrochemical cell. It ensures that electrons lost at the anode are replenished at the cathode, allowing the electrochemical reactions to proceed smoothly.
Without the salt bridge, the electrochemical cell would be incomplete, and the flow of current would be hindered. It is the unsung hero that connects the anode and cathode, providing a pathway for ion exchange and maintaining electrical neutrality, making electrochemical reactions possible and unlocking their potential in various technological applications.
Oxidation and Reduction: The Heart of Electrochemical Cells
In the realm of electrochemical cells, a salt bridge plays a crucial role in enabling the dance of electrons, driving chemical reactions that shape our world. At the heart of these cells lies a fundamental concept known as oxidation-reduction, where electrons are exchanged like partners in a cosmic ballet.
Imagine two compartments, separated by a porous barrier but connected by the ethereal presence of ions flowing through a salt bridge. Within each compartment, chemical reactions unfold, driven by an electrical potential difference. In one compartment, oxidation takes center stage: electrons leap from atoms, leaving behind positively charged ions. This is akin to a shy performer stepping into the spotlight, shedding their timid facade.
In perfect harmony, electrons waltz across the salt bridge to the other compartment, where reduction unfolds. Here, electrons embrace atoms, transforming them into negatively charged ions. It’s like a welcoming embrace, granting these atoms a newfound energy.
The salt bridge, acting as a bridge of electrons, allows this intricate exchange to take place. It facilitates the flow of ions between compartments, ensuring that the dance of oxidation and reduction can continue, fueling electrochemical reactions that power our lives.
The Salt Bridge: Completing the Circuit in Electrochemical Cells
Unveiling the Mysteries of Electrochemical Circuits
In the realm of electrochemistry, a salt bridge plays a pivotal role in orchestrating the flow of ions and electrons, completing the vital circuit that drives electrochemical reactions. Imagine an electrochemical cell, a device that converts chemical energy into electrical energy or vice versa. Within this cell, a salt bridge serves as the unseen conductor, facilitating the movement of ions between two electrolytic solutions, allowing for the completion of the circuit and the occurrence of electrochemical reactions.
Defining the Electrochemical Circuit
An electrochemical circuit is a closed loop that allows for the flow of current. It consists of several key components:
- Power source: Provides the electrical energy to drive the electrochemical reaction.
- Electrodes: Conductors that connect the circuit to the electrolytic solutions. One electrode undergoes oxidation (loss of electrons), while the other undergoes reduction (gain of electrons).
- Electrolytic solutions: Contain ions that can move freely, allowing for the conduction of electricity.
- Salt bridge: Connects the two electrolytic solutions, enabling the flow of ions to maintain electrical neutrality.
The Salt Bridge: Completing the Loop
The salt bridge is a key component that completes the electrochemical circuit. It provides a pathway for ions to flow between the two electrolytic solutions. This ion flow is essential for maintaining electrical neutrality within the cell. Without the salt bridge, ions would accumulate in one solution and deplete in the other, disrupting the balance of charge and preventing the flow of current.
Ensuring Electrical Neutrality
Electrical neutrality is crucial for the proper functioning of an electrochemical cell. The salt bridge helps maintain this neutrality by allowing ions to move between the two electrolytic solutions. As ions are consumed or produced during electrochemical reactions, the salt bridge ensures that the overall charge balance is maintained. This allows for the continuous flow of current and the sustained occurrence of electrochemical reactions.
Promoting Electrochemical Reactions
The flow of ions through the salt bridge facilitates electrochemical reactions. Oxidation occurs at the anode (negative electrode), where electrons are lost and positive ions are produced. These positive ions migrate through the salt bridge to the cathode (positive electrode), where they undergo reduction and gain electrons. This movement of ions and electrons completes the circuit and drives the electrochemical reaction.
The salt bridge plays an indispensable role in electrochemical circuits, enabling the flow of ions, maintaining electrical neutrality, and facilitating electrochemical reactions. It completes the loop, allowing for the conversion of chemical energy into electrical energy or vice versa. Without the salt bridge, electrochemical cells would be unable to function, highlighting its critical importance in the world of electrochemistry.