Revisiting The Miller-Urey Experiment: Uncovering Limitations And Unveiling Abiogenesis Complexities
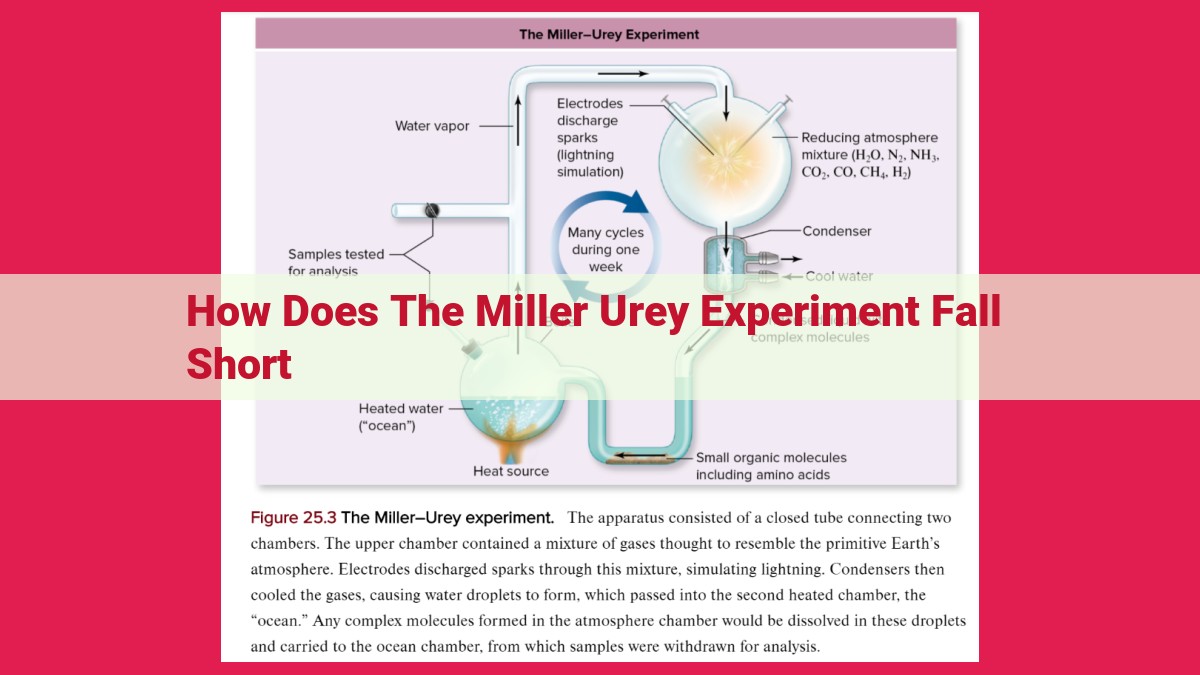
The Miller-Urey experiment, while groundbreaking, had several limitations: it lacked nitrogen oxide (NO) and UV radiation, essential for amino acid and nucleotide formation. The simplified gas mixture and absence of liquid water hindered complex molecule synthesis. Additionally, the rapid reaction time and intermittent energy input did not fully replicate Earth’s early atmosphere. These limitations necessitate further research and more comprehensive atmospheric simulations to better understand abiogenesis.
The Importance of Nitrogen Oxide (NO) in the Miller-Urey Experiment
In the realm of scientific exploration, the Miller-Urey experiment has left an enduring mark on our understanding of abiogenesis. This groundbreaking experiment aimed to simulate the conditions of Earth’s early atmosphere to shed light on the origins of life. However, despite its pivotal role, the experiment fell short in one crucial aspect: the lack of nitrogen oxide (NO).
Nitrogen oxide is an essential ingredient for the formation of amino acids, the building blocks of proteins, and nucleotides, the key components of DNA and RNA. These vital molecules are necessary for the existence of life as we know it. NO plays a critical role in driving the chemical reactions that produce these essential compounds.
In Earth’s primordial atmosphere, nitrogen oxide was abundant. It is a powerful oxidizer that can break down complex molecules into simpler components. This process releases energy, which can then be harnessed to drive the formation of amino acids and nucleotides. The absence of NO in the Miller-Urey experiment, therefore, significantly limited the synthesis of these essential biomolecules.
How the Miller-Urey Experiment Falls Short: Lack of Nitrogen Oxide
The Miller-Urey experiment, a landmark study in the field of abiogenesis, simulated the conditions of Earth’s early atmosphere to investigate the formation of organic molecules. However, the experiment had several limitations, one of which was the absence of nitrogen oxide (NO).
Nitrogen oxide is a crucial component in the formation of amino acids and nucleotides, the essential building blocks of proteins and DNA, respectively. In the early atmosphere, NO was produced by electrical discharges, such as lightning.
Without NO, the Miller-Urey experiment significantly underestimated the synthesis of amino acids and nucleotides. The experiment produced only 11 out of the 20 common amino acids found in proteins and failed to produce any nucleotides.
The lack of NO limited the potential for the formation of more complex organic molecules. Amino acids and nucleotides are essential for the structure and function of proteins and DNA, which play vital roles in the processes of life. The absence of NO in the Miller-Urey experiment, therefore, hindered our understanding of how the building blocks of life could have arisen in the early Earth’s atmosphere.
The Unsung Hero of Organic Origin: The Role of UV Radiation in the Miller-Urey Experiment
Imagine the primordial Earth as a vast laboratory teeming with an exotic mixture of gases. Amidst this cosmic crucible, the Miller-Urey experiment sought to recreate the conditions believed to have given rise to life. However, one crucial ingredient was missing: the omnipresent energy of UV radiation.
UV radiation serves as an invisible maestro, guiding the dance of molecules in the atmosphere. Its high-energy photons possess the ability to break down gases into simpler components, creating a sea of reactive chemical building blocks.
In the Miller-Urey experiment, the absence of UV radiation hindered the formation of complex organic molecules. Without this celestial spark, the gases remained relatively stable, unable to fully embrace the transformative power of chemical reactions. It’s as if a symphony of molecular interactions was missing its conductor, leaving the potential for life unrealized.
Consider the synthesis of amino acids, the fundamental building blocks of proteins. UV radiation catalyzes this process by breaking down nitrogen molecules into nitrogen atoms, which can then combine with other molecules to form these essential organic compounds. Similarly, the formation of nucleotides, the building blocks of DNA, requires the energy input of UV radiation to break down and rearrange molecules.
The lack of UV radiation in the Miller-Urey experiment diminished the synthesis of these vital molecules, hampering the potential for the emergence of life. It’s like trying to bake a cake without an oven—the essential ingredients are present, but the spark of transformation is absent.
As we delve deeper into the mysteries of abiogenesis, it’s crucial to acknowledge the shortcomings of the Miller-Urey experiment. By understanding the vital role of UV radiation and other factors that were not fully accounted for, we can refine our understanding of the conditions that gave rise to life on Earth.
The Miller-Urey Experiment: Unveiling Its Limitations
The Miller-Urey experiment, conducted in 1953, remains a landmark study in the field of abiogenesis. However, its limitations have raised questions about its accuracy in portraying the conditions that may have led to the origin of life on Earth. One significant limitation is the absence of ultraviolet (UV) radiation in the experiment.
UV radiation plays a crucial role in breaking down gases and initiating chemical reactions. In the early Earth’s atmosphere, UV radiation from the sun would have been a prominent source of energy. The lack of UV radiation in the Miller-Urey experiment hindered the formation of complex organic molecules by limiting the breakdown of gases and the energy available for chemical reactions.
As a result, the experiment produced a limited variety and quantity of organic molecules compared to what may have been present in the actual early Earth’s atmosphere. The absence of UV radiation also affected the stability of these molecules, as UV radiation can act as a sterilizing agent, preventing their degradation and allowing for their accumulation over time.
In conclusion, the lack of UV radiation in the Miller-Urey experiment significantly underrepresented the conditions under which organic molecules may have formed on early Earth. This limitation highlights the need for further research and more comprehensive atmospheric simulations to fully understand the complex processes involved in abiogenesis.
How the Miller-Urey Experiment Falls Short: Unveiling the Limitations of Abiogenesis Research
The Miller-Urey experiment, conducted in 1953, played a pivotal role in advancing our understanding of the origin of life. However, it’s crucial to acknowledge its limitations, which became apparent as scientists delved deeper into the complexities of Earth’s early atmosphere.
Limited Variety of Gases Used
One significant limitation of the Miller-Urey experiment lay in the simplified mixture of gases it employed. This mixture primarily consisted of methane, ammonia, water vapor, and hydrogen, based on the limited knowledge of Earth’s early atmosphere at the time. However, subsequent research has revealed that the Earth’s atmosphere was likely much more complex, containing a wide range of gases, including nitrogen, carbon dioxide, and sulfur compounds.
Impact of Omitted Gases
The absence of these omitted gases had profound implications for the experiment’s results. For example, nitrogen is a crucial component for the synthesis of amino acids and nucleotides, the essential building blocks of proteins and DNA. Carbon dioxide plays a role in the formation of sugars, another fundamental component of life. Sulfur compounds, such as hydrogen sulfide, could have acted as a source of energy and a precursor to the formation of organic molecules.
The exclusion of these gases from the Miller-Urey experiment led to an incomplete representation of the chemical conditions that existed on early Earth.
Oversimplified Atmosphere
Another limitation was the oversimplified representation of the early atmosphere. The experiment did not account for the variations in temperature, pressure, and humidity that would have existed in the real environment. Additionally, the experiment did not simulate the presence of dust, aerosols, or minerals, which could have played significant roles in the formation of organic molecules.
These oversimplifications limited the experiment’s ability to accurately replicate the complex interactions that occurred in the early Earth’s atmosphere and may have contributed to the incomplete synthesis of organic compounds.
Limited Variety of Gases Used
In the Miller-Urey experiment, only four gases were used to simulate Earth’s early atmosphere: methane (CH4), ammonia (NH3), water (H2O), and hydrogen (H2). While these gases were certainly present, the Earth’s early atmosphere was a far more complex mixture.
Research suggests that other gases, such as carbon monoxide (CO), carbon dioxide (CO2), nitrogen oxide (NO), and hydrogen sulfide (H2S), were also present. These additional gases could have played a crucial role in the formation of organic molecules.
Carbon monoxide and carbon dioxide, for example, are essential components for the synthesis of nucleotides, the building blocks of DNA. Nitrogen oxide is involved in the formation of amino acids, the building blocks of proteins. Hydrogen sulfide may have provided sulfur atoms for the formation of organic molecules.
By simplifying the atmosphere to a limited number of gases, the Miller-Urey experiment may have underestimated the potential for organic molecule formation. Including a more chemically diverse atmosphere in future experiments would provide a more accurate representation of Earth’s early conditions.
How the Miller-Urey Experiment Falls Short
Lack of Gases: Oversimplified Atmosphere
The Miller-Urey experiment used a simplified mixture of gases, mainly methane (CH4), ammonia (NH3), water vapor (H2O), and hydrogen (H2), to simulate Earth’s early atmosphere. However, scientists now believe that Earth’s early atmosphere was much more complex, containing additional gases such as carbon monoxide (CO), carbon dioxide (CO2), hydrogen sulfide (H2S), and nitrogen dioxide (NO2).
The Importance of Nitrogen Dioxide
Nitrogen dioxide (NO2) is crucial for the formation of amino acids and nucleotides, which are the building blocks of proteins and DNA. In the Miller-Urey experiment, the absence of NO2 limited the synthesis of these essential molecules.
Other Excluded Gases
Other gases like sulfur dioxide (SO2), phosphine (PH3), and hydrogen cyanide (HCN) were also missing from the experimental setup. These gases are believed to have played significant roles in prebiotic chemistry and may have contributed to the formation of complex organic molecules.
Implications for Abiogenesis
The limited variety of gases used in the Miller-Urey experiment means that its results may not accurately represent the conditions under which organic molecules first formed on Earth. More comprehensive atmospheric simulations that include a wider range of gases are needed to refine our understanding of abiogenesis, the process by which life arose from non-living matter.
How the Miller-Urey Experiment Falls Short: A Deeper Dive into Its Limitations
In 1953, the groundbreaking Miller-Urey experiment sparked excitement in the scientific community, suggesting the possibility of forming organic molecules from inorganic materials. However, as research progressed, scientists uncovered several limitations that cast doubt on its ability to fully replicate the conditions of Earth’s early atmosphere.
Oversimplified Atmosphere
The gases used in the Miller-Urey experiment were a simplified representation of the complex atmospheric composition during Earth’s early years. Gases like sulfur dioxide (SO2) and ammonia (NH3), which could have contributed to prebiotic chemistry, were notably absent.
Consequences: These gases are critical for the formation of certain amino acids and nucleotides, essential building blocks of proteins and DNA. By excluding them, the experiment missed out on a significant portion of potential organic molecule synthesis.
Additionally, the experiment’s composition assumed a constant, uniform atmosphere, ignoring the likely variations in gas concentrations and atmospheric layers present in prebiotic Earth. This oversimplification may have skewed the types and quantities of organic molecules produced.
Lack of Liquid Water
Liquid water, a crucial solvent for chemical reactions, was absent in the Miller-Urey experiment. While water vapor was present, it could not effectively facilitate the formation and stability of complex organic molecules.
Consequences: The absence of liquid water hindered the stability of organic compounds. Without a medium to dissolve and disperse them, molecules would have struggled to interact, limiting the chances for further reactions and molecular growth. Additionally, liquid water can prevent the breakdown of organic molecules by UV radiation, further reducing their chances of survival.
The Miller-Urey Experiment: Falling Short in the Search for Life’s Origins
The Miller-Urey experiment, conducted in 1953, was a landmark study that attempted to simulate the conditions on early Earth to investigate the possibility of abiogenesis, the formation of life from non-living matter. While the experiment yielded promising results, it faced several limitations that raised questions about its accuracy and completeness.
5. The Missing Link: Liquid Water
One crucial oversight in the Miller-Urey experiment was the absence of liquid water. Water is the universal solvent, essential for countless chemical reactions and the stability of organic molecules. Without it, the formation and survival of complex organic molecules, the building blocks of life, was severely hindered.
Water plays a multifaceted role in chemical reactions. It acts as a reaction medium, enabling molecules to interact and form new bonds. It also facilitates the transfer of heat and energy, providing the conditions necessary for chemical transformations. Additionally, water serves as a stabilizing force for organic molecules, protecting them from degradation and preserving their structure.
In the Miller-Urey experiment, the lack of liquid water limited the scope of chemical reactions and the stability of any organic molecules that may have formed. Without a suitable solvent, the formation of complex organic molecules, such as amino acids and nucleotides, was significantly reduced. This deficiency casts doubt on the experiment’s ability to accurately simulate the conditions on early Earth, where liquid water is believed to have been abundant.
How the Miller-Urey Experiment Falls Short: The Absence of Liquid Water
The Miller-Urey experiment, a landmark study in the field of abiogenesis, sought to recreate the conditions of Earth’s early atmosphere and investigate the potential for the formation of organic molecules. However, one glaring omission in the experiment was the absence of liquid water.
Liquid Water: An Essential Solvent
Water is an essential solvent for chemical reactions. It provides a medium in which molecules can dissolve and interact with each other. Without water, many of the chemical reactions necessary for the formation of complex organic molecules would simply not occur. For example, the formation of amino acids, the building blocks of proteins, requires water as a solvent.
Stability in Aqueous Environments
Water also provides a stable environment for organic molecules once they have formed. Organic molecules, particularly complex ones like proteins and DNA, are fragile and can easily be broken down by factors such as heat and radiation. Water can shield these molecules from such harmful agents and allow them to persist for longer periods. In the absence of liquid water, it is less likely that complex organic molecules could have formed and survived in Earth’s early atmosphere.
Implications for Abiogenesis
The omission of liquid water in the Miller-Urey experiment is a significant limitation in our understanding of abiogenesis. It suggests that the conditions under which organic molecules could have formed on early Earth may have been more complex than originally thought. Further research and more comprehensive atmospheric simulations that include liquid water are necessary to refine our knowledge and gain a deeper understanding of the origins of life.
How the Miller-Urey Experiment Falls Short
In the quest to unravel the origins of life, the Miller-Urey experiment has left an enduring mark. However, as our understanding of the conditions that prevailed on early Earth has evolved, scientists have identified limitations in the experiment that raise questions about its conclusions.
6. Rapid Reaction Time:
The Miller-Urey experiment simulated lightning by delivering high-frequency electrical sparks to a sealed flask containing water, methane, ammonia, and hydrogen. These sparks were brief and intermittent, providing a sudden burst of energy. However, on early Earth, lightning was a constant and continuous source of energy, lasting for extended periods. This prolonged energy input would have allowed more time for complex organic molecules to form and interact with each other.
The experiment’s short-lived bursts of energy may have led to the premature termination of chemical reactions, preventing the formation of more complex and stable molecules. This limitation highlights the need for more sophisticated experiments that can replicate the continuous and prolonged energy input that characterized the early Earth’s atmosphere.
By understanding the limitations of the Miller-Urey experiment, scientists can better design future experiments that more accurately reflect the conditions that existed on early Earth. This will help us refine our knowledge of the abiogenesis, the process by which life arose from non-living matter.
The Miller-Urey Experiment: A Spark of Insight and a Glimmer of Doubt
Dr. Stanley Miller and Dr. Harold Urey, pioneers in the field of abiogenesis, embarked on a groundbreaking experiment in 1953. Their goal was to simulate the conditions on early Earth and investigate the possibility of synthesizing organic molecules from inorganic matter. The Miller-Urey experiment became iconic, but its limitations have also shed light on the complexities of the origin of life.
The Role of Lightning: A Fleeting Spark
One key limitation of the Miller-Urey experiment was the rapid reaction time afforded by the use of high-frequency electrical sparks to simulate lightning. These sparks, while mimicking the energy input of lightning, only lasted for brief periods. This limited the time available for complex organic molecules to form and interact with each other.
Nature’s Continuous Symphony: Missing from the Experiment
In Earth’s early atmosphere, energy was constantly supplied by sources such as lightning and UV radiation. However, the Miller-Urey experiment only provided intermittent and short-lived energy bursts. This lack of continuous energy input hindered the formation of larger and more complex organic molecules as they required sustained exposure to energy sources to assemble and stabilize.
Time, a Crucial Element: Lost in the Sparks
The rapid reaction time and the lack of sustained energy input meant that the Miller-Urey experiment was unable to fully capture the extended time scales necessary for complex organic molecules to emerge. The experiment essentially provided a glimpse into the initial stages of organic molecule formation but fell short of replicating the conditions that may have prevailed over millions of years on early Earth.
How the Miller-Urey Experiment Falls Short: Unlocking the Secrets of Life’s Origins
The Miller-Urey Experiment: A Landmark Study with Limitations
The Miller-Urey experiment, conducted in 1953, was a groundbreaking experiment that attempted to simulate the conditions of Earth’s early atmosphere and demonstrate the spontaneous formation of organic molecules from inorganic precursors. While the experiment provided valuable insights, it also had limitations that have shaped our understanding of abiogenesis.
The Continuous Energy Input: A Vital Force in Earth’s Early Atmosphere
One crucial aspect that the Miller-Urey experiment lacked was continuous energy input. Earth’s early atmosphere was constantly bombarded with ultraviolet radiation from the sun and lightning strikes from thunderstorms. These energy sources provided the driving force for chemical reactions that could produce organic molecules.
- Ultraviolet radiation: This high-energy radiation can break down molecules in the atmosphere, creating free radicals that can react with other molecules to form more complex compounds.
- Lightning strikes: These powerful electrical discharges can release massive amounts of energy in the form of heat, light, and electrical currents. This energy can ionize molecules, making them more reactive and facilitating chemical reactions.
The Importance of Continuous Energy Input
The Miller-Urey experiment relied on brief and intermittent electrical sparks to simulate lightning. However, this did not fully replicate the continuous and sustained energy input that was present in Earth’s early atmosphere. As a result, the experiment may have underestimated the potential for the formation of complex organic molecules.
Additionally, the lack of liquid water in the experiment hindered the stability and survival of organic molecules. The presence of liquid water is essential for many chemical reactions and provides a stable environment for organic molecules to form and accumulate.
Implications for Abiogenesis
The limitations of the Miller-Urey experiment highlight the need for further research and more comprehensive atmospheric simulations to refine our understanding of abiogenesis. By accounting for the continuous energy input and other factors, we can gain a more accurate picture of the conditions that may have led to the spontaneous emergence of life on Earth.
How the Miller-Urey Experiment Falls Short: Unveiling the Missing Pieces
The Miller-Urey experiment, conducted in 1953, was a groundbreaking attempt to simulate the conditions of Earth’s early atmosphere and explore the potential for spontaneous chemical reactions that could lead to the formation of organic molecules. While it provided valuable insights, subsequent research has revealed several key limitations that hinder our full understanding of abiogenesis.
Lack of Continual Energy Input: A Crucial Omission
One significant shortcoming of the Miller-Urey experiment was the intermittent and brief energy input. Natural conditions on early Earth were characterized by continuous energy input from lightning, UV radiation, and other sources. However, the experiment used only a brief burst of electrical sparks to simulate lightning, which provided insufficient energy for complex chemical reactions.
This limited energy duration hindered the formation of more intricate organic molecules because these reactions typically require sustained and prolonged energy input. In nature, the continuous bombardment of high-energy lightning or intense UV radiation would have provided a more conducive environment for the synthesis of complex molecules, promoting bond formation and rearrangements over an extended period.
Summarize the limitations of the Miller-Urey experiment and their implications for our understanding of abiogenesis.
How the Miller-Urey Experiment Falls Short: Unraveling the Mysteries of Abiogenesis
The Miller-Urey experiment, a groundbreaking endeavor in the field of abiogenesis, sought to simulate conditions believed to exist on early Earth and explore the potential for spontaneous organic molecule formation. However, despite its significance, the experiment faced several limitations that hindered its ability to provide a comprehensive account of the origin of life.
1. Absence of Nitrogen Oxide (NO)
Nitrogen oxide (NO) is a crucial component in the synthesis of amino acids and nucleotides, the building blocks of proteins and DNA, respectively. However, the Miller-Urey experiment lacked NO, significantly limiting the formation of these essential molecules.
2. Lack of UV Radiation
Ultraviolet radiation played a pivotal role in breaking down gases and promoting chemical reactions on early Earth. Its absence in the experiment hindered the formation of complex organic molecules, as UV radiation’s energy was not available to drive these reactions.
3. Limited Variety of Gases Used
The Miller-Urey experiment employed a simplified mixture of gases that did not fully represent the complex composition of Earth’s early atmosphere. This oversimplification may have resulted in the exclusion of gases that could have contributed to organic molecule formation.
4. Oversimplified Atmosphere
The experiment’s simulated atmosphere lacked several gases that could have played a significant role in abiogenesis, such as hydrogen sulfide (H2S) and ammonia (NH3). The absence of these gases could have influenced the formation and stability of organic molecules.
5. Lack of Liquid Water
Liquid water is essential for chemical reactions and the stability of organic molecules. The Miller-Urey experiment was conducted in a sealed apparatus without liquid water, potentially constraining the formation and survival of complex organic compounds.
6. Rapid Reaction Time
The experiment simulated lightning using high-frequency electrical sparks. However, these sparks provided a short-lived energy input, limiting the time available for complex organic molecules to form and stabilize.
7. Lack of Continual Energy Input
In Earth’s early atmosphere, energy input from sources like lightning and UV radiation was continuous. The experiment’s brief and intermittent energy input did not accurately replicate these conditions, which could have hindered the synthesis of complex organic molecules.
While the Miller-Urey experiment provided insights into the potential for abiogenesis, its limitations necessitate further research and more comprehensive atmospheric simulations. These limitations underscore the complexity of abiogenesis and the need for a nuanced understanding of the conditions that may have led to the emergence of life on Earth.
Emphasize the need for further research and more comprehensive atmospheric simulations to refine our knowledge.
How the Miller-Urey Experiment Sparks Curiosity but Falls Short
The Miller-Urey experiment, a groundbreaking experiment conducted in 1953, captivated the scientific community with its exploration of the origin of life on Earth. While it demonstrated the potential for abiogenesis, it also revealed limitations that continue to fuel research today.
Limitations that Highlight the Need for Further Exploration
The Miller-Urey experiment lacked nitrogen oxide, an essential catalyst for the formation of amino acids and nucleotides. The absence of UV radiation, a powerful driver of chemical reactions, hindered the conversion of gases into complex organic molecules. The experiment’s simplistic atmosphere, which excluded gases present in Earth’s early environment, likely influenced the results.
The Complexity of Earth’s Atmosphere
Earth’s early atmosphere was a complex mixture of gases, including methane, ammonia, hydrogen, and carbon dioxide. The experiment’s atmosphere lacked several key gases, potentially skewing the synthesis of organic molecules.
The Missing Element: Liquid Water
Liquid water provides a stable environment for chemical reactions and supports the solubility of organic molecules. The experiment’s absence of liquid water prevented the formation and preservation of complex molecules.
The Challenges of Energy Input
The experiment used high-frequency electrical sparks to simulate lightning. However, these sparks provided only brief and intermittent energy input, unlike the continuous energy sources (e.g., lightning and UV radiation) present in Earth’s early atmosphere.
Moving Forward with Comprehensive Research
The Miller-Urey experiment remains a landmark achievement, but its limitations highlight the complexity of abiogenesis. Future research must explore these limitations by employing more comprehensive atmospheric simulations. By addressing the shortcomings of the Miller-Urey experiment, we can refine our understanding of the origin of life on Earth and the potential for life beyond our planet.
Additional Research Avenues
- Exploring the role of nitrogen oxide and other missing gases in organic molecule synthesis
- Simulating the continuous energy input of Earth’s early atmosphere
- Investigating the effects of liquid water on the stability and complexity of organic molecules
- Developing more sophisticated atmospheric models to accurately replicate Earth’s early conditions
These research avenues will not only enhance our knowledge of abiogenesis but also provide valuable insights into the potential for life on other planets in our vast and enigmatic universe.