Unveiling The Rate-Limiting Step: A Key Factor In Comprehending Reaction Rates
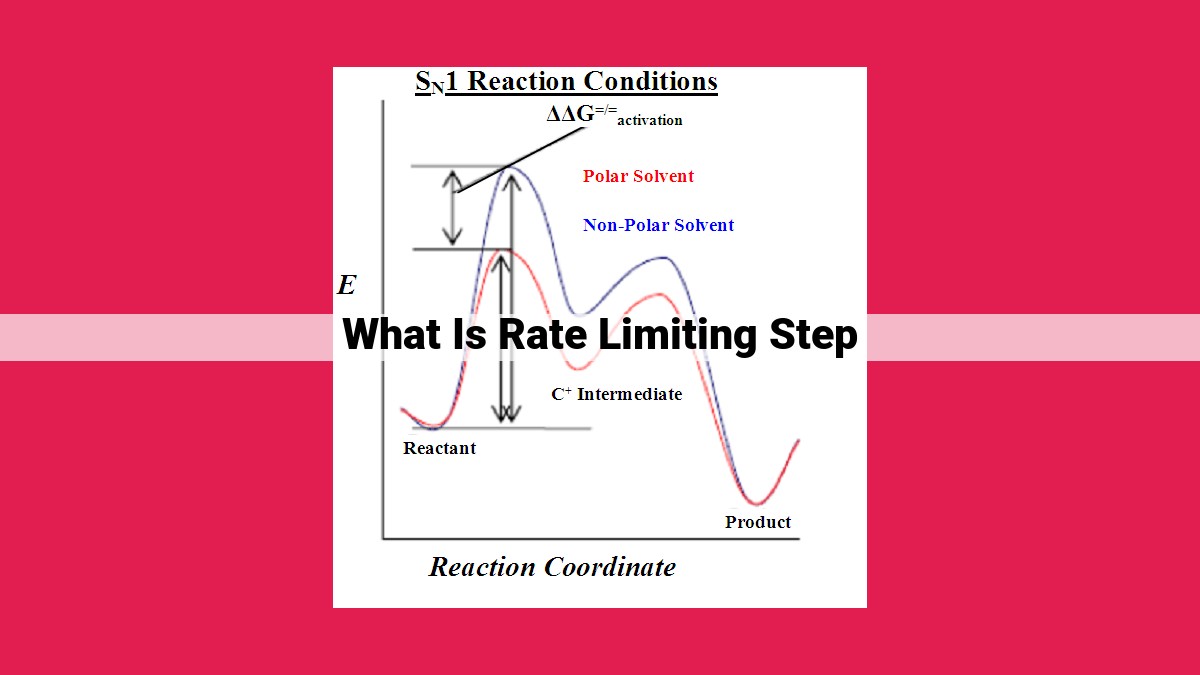
The rate-limiting step is the slowest step in a reaction sequence, determining the overall reaction rate. It depends on the activation energy, which is related to the frequency and energy of collisions between reactants. Collision theory and Arrhenius equation describe this relationship. Intermediates and transition states play crucial roles in reaction mechanisms, with Hammond’s postulate linking transition states to product structure. Understanding the rate-limiting step is essential for comprehending reaction mechanisms and the factors that govern reaction rates.
The Rate-Limiting Step: Understanding the Pacemaker of Chemical Reactions
In the realm of chemical reactions, there exists a critical concept known as the rate-limiting step. This concept is akin to identifying the bottleneck in a production line, a crucial step that determines the overall pace of the entire process. Just as a slow-moving assembly line worker can hinder production, a slow rate-limiting step can significantly impact the reaction rate, the speed at which reactants transform into products.
Comprehending the rate-limiting step is not merely an academic exercise; it is a fundamental pillar in unraveling the intricate mechanisms of chemical reactions. By identifying the slowest step in a reaction sequence, chemists can gain insights into the molecular dance that leads to the formation of new substances. This knowledge empowers them to manipulate reaction conditions, such as temperature and concentration, to optimize reaction rates and achieve desired outcomes.
Understanding Related Concepts
In the realm of chemistry, understanding the rate-limiting step is crucial to deciphering the tapestry of chemical reactions. Related concepts such as reaction rate and kinetics dance around this central stage, illuminating the intricate mechanisms that govern the speed and pathways of reactions.
Reaction Rate: The Swiftness of Change
The reaction rate embodies the change in concentration of reactants or products over time, serving as a direct measure of a reaction’s velocity. This metric reflects the rate-limiting step‘s profound influence, as the slowest step dictates the overall pace of the reaction.
Kinetics: Unraveling the Dynamics
Kinetics is the science that delves into the intricacies of reaction rates and their dependency on external factors such as temperature and concentration. By peering into the kinetic landscape, chemists can unearth the underlying mechanisms, discover activation energies, and predict the behavior of reactions under different conditions.
These concepts intertwine, forming an indispensable web of knowledge that unravels the secrets of chemical transformations. The rate-limiting step, as the heart of this intricate dance, orchestrates the tempo of reactions, shaping the path from reactants to products with precision and elegance.
Unlocking the Secrets of Chemical Reactions: The Role of Activation Energy
Imagine a chemical reaction as a race, with reactants as the eager runners and products as the finish line. However, there’s a hurdle they must overcome — activation energy, the energy required to reach the transition state, a high-energy peak they must cross to complete the reaction.
Activation energy acts like a bouncer at an exclusive club, strictly controlling who gets to the dance floor. Only reactants with enough energy can reach the transition state and dance on to become products. The higher the activation energy, the slower the reaction.
Scientists use the Arrhenius equation to express this relationship mathematically:
k = Ae^(-Ea/RT)
Where:
- k is the reaction rate
- A is the pre-exponential factor
- Ea is the activation energy
- R is the gas constant
- T is the temperature
As temperature increases, more reactant molecules have enough energy to reach the transition state, speeding up the reaction.
Another key concept is collision theory, which suggests that reactions occur when reactants collide with enough energy and in the correct orientation. The more frequent and energetic these collisions, the faster the reaction.
In summary, activation energy is a crucial factor that determines how quickly a chemical reaction occurs. By understanding its role, we can better predict and control chemical processes, paving the way for advancements in fields like medicine, energy, and materials science.
The Significance of Intermediates: Unveiling the Hidden Players in Chemical Reactions
In the intricate dance of chemical reactions, there are more players involved than meet the eye. Intermediates, fleeting and enigmatic species, emerge as key participants, shaping the very essence of these transformations.
Intermediates: The Unsung Heroes of Chemistry
Intermediates are temporary molecules that grace the stage of a reaction for a brief moment. They exist only during the reaction’s progression, their existence hidden from the final curtain call. Unlike the reactants and products, intermediates do not appear in the overall reaction equation.
Reaction Mechanisms: A Behind-the-Scenes Glimpse
Understanding intermediates and their role requires delving into reaction mechanisms. These detailed accounts unveil the intricate steps of a reaction, illuminating the sequence of events that lead from reactants to products. Intermediates take center stage in these mechanisms, showcasing their crucial involvement.
Catalytic Cycles: Intermediates Take the Stage
Certain reactions can’t resist the allure of catalysts, substances that accelerate their pace. Catalysts engage in an intimate dance with intermediates, forming and breaking bonds with them. This catalytic cycle breathes life into reactions, making them proceed at a quicker tempo.
In conclusion, intermediates, though seemingly ephemeral, play an indispensable role in chemical reactions. Their presence in reaction mechanisms and their participation in catalytic cycles underscores their significance. Understanding intermediates grants us a deeper insight into the dynamic symphony of chemical transformations.
Entering the Quantum Realm: Unraveling the Enigmatic Nature of Transition States
Defining the Elusive Gateways
Transition states lurk within the intricate tapestry of chemical reactions, representing fleeting yet pivotal moments where reactants teeter on the cusp of transformation. These high-energy peaks, often referred to as activated complexes, serve as inescapable gateways that molecules must traverse to complete their chemical journeys.
Hammond’s Wisdom: Predicting the Transition State’s Disguise
Enter Hammond’s postulate, an insightful principle that unveils a hidden connection between transition states and products. It whispers, “the transition state resembles the structure of the product that lies just beyond its grasp.” By peering into the mirror of the transition state, chemists gain a glimpse of the molecular future that awaits.
Marcus Theory: Electron Transfer’s Elusive Dance
For electron transfer reactions, where electrons leap from one molecule to another, Marcus theory takes center stage. Like a maestro orchestrating a symphony, this theory describes the delicate balance between energy levels, guiding electrons towards their new homes. The closer the energy gap between reactants and products, the smoother the electron’s transition.
Transition states are not mere spectators in the realm of chemical reactions. They are active participants, shaping the reaction’s destiny and revealing the intricate dance between reactants, products, and the elusive quantum world that governs their interactions. By understanding these enigmatic states, we unlock the secrets of chemical transformations and glimpse the hidden forces that drive the molecular universe.