Radiometric Dating: Unlocking The Secrets Of Earth’s Past
Radiometric dating, a technique exploiting radioactive decay, is used to determine the age of sedimentary rocks. It relies on the accumulation of stable daughter isotopes as parent isotopes decay at a constant rate, governed by their half-life. Comparing the proportions of parent and daughter isotopes allows scientists to calculate the age of the rock. This technique has revolutionized geology, enabling the dating of formations, reconstructing past environments, and establishing the Earth’s geological timeline.
Embark on an extraordinary journey through the annals of time, unlocking the secrets of Earth’s past with the power of radiometric dating. This technique unveils the age of sedimentary rocks, unveiling the tales they hold about our planet’s evolution.
Radiometric dating is a scientific method that harnesses the natural decay of radioactive isotopes to determine the age of rocks. It’s like peering through a time capsule, allowing geologists to decipher the chronology of sedimentary layers that have accumulated over millions of years. By measuring the ratio of parent isotopes (the unstable elements that decay) to daughter isotopes (the stable elements they transform into), scientists can calculate the elapsed time since the rock formed.
This breakthrough has revolutionized our understanding of Earth’s history, providing a reliable timeline against which we can measure the rise and fall of ancient civilizations, the shifting of continents, and the evolution of life itself.
Fundamentals of Radioactive Decay
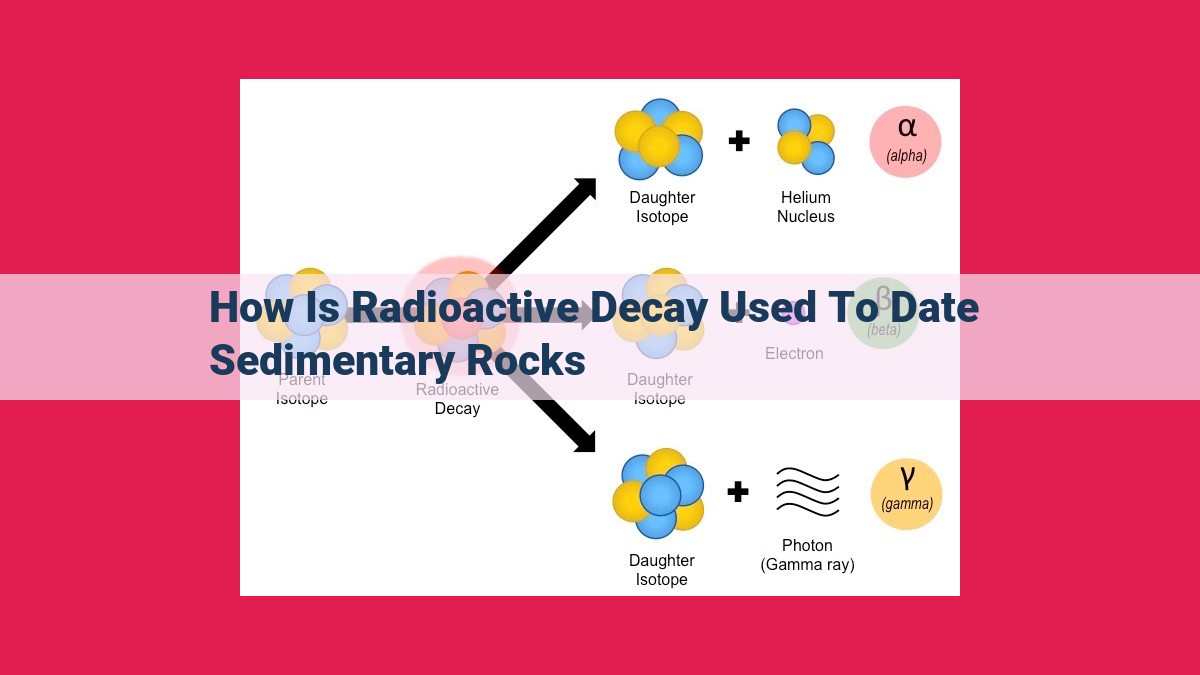
- Describe the three types of radioactive decay (alpha, beta, and gamma) and how they occur.
- Define half-life and its significance in dating rocks.
Unveiling Earth’s Secrets with Radioactive Decay
Fundamentals of Radioactive Decay: The Unsung Storytellers of Time
To unravel the secrets of Earth’s past, we turn to the enigmatic world of radioactive decay, a natural phenomenon that holds the key to deciphering the age of sedimentary rocks. These rocks, formed by the accumulation of sediments over time, contain hidden treasures that reveal their age and the history of our planet.
Radioactive decay involves the spontaneous transformation of an unstable parent isotope into a more stable daughter isotope, releasing energy in the form of radiation. There are three main types of radioactive decay:
- Alpha decay: The emission of a helium nucleus, consisting of two protons and two neutrons.
- Beta decay: The conversion of a neutron into a proton or vice versa, resulting in the emission of a beta particle (electron or positron).
- Gamma decay: The emission of high-energy photons, similar to X-rays, but without any change in the atomic structure.
Each parent isotope has a unique half-life, which is the time it takes for half of the parent atoms to decay into daughter atoms. This half-life is crucial in determining the age of rocks because it provides a yardstick against which the relative abundance of parent and daughter isotopes can be measured.
As the parent isotope decays, the daughter isotope accumulates in the rock. By measuring the ratio of parent to daughter isotopes and knowing the half-life of the parent isotope, scientists can calculate the age of the rock. This technique, known as radiometric dating, has revolutionized our understanding of Earth’s history and the timeline of geological events.
Parent and Daughter Isotopes in Sedimentary Rocks
Every atom comprises a nucleus, which contains protons and neutrons, and electrons that orbit around it. Certain elements have isotopes, variations of the same element with differing numbers of neutrons. Radioactive isotopes are unstable isotopes that decay over time, emitting energy in the process.
Parent isotopes are the radioactive isotopes that undergo decay. Daughter isotopes are the stable isotopes produced as a result of this decay. The parent and daughter isotopes are closely linked; the parent isotope decays directly to form the daughter isotope.
In sedimentary rocks, parent isotopes are present in minerals deposited during the rock’s formation. Over time, the parent isotopes decay, transforming into daughter isotopes. This process is ongoing, and the ratio of parent to daughter isotopes in a rock changes gradually.
This gradual change in the ratio of parent to daughter isotopes forms the basis of radiometric dating. By measuring the ratio of these isotopes and knowing the half-life (the time it takes for half of the parent isotopes to decay) of the parent isotope, scientists can determine the age of the sedimentary rock.
So, in sedimentary rocks, parent isotopes act as “timekeepers,” steadily decaying into daughter isotopes. This decay process provides valuable insights into the geological history and time scales of Earth’s sedimentary formations.
Radiometric Dating of Sedimentary Rocks: Unlocking Earth’s Time Capsule
A Journey into Earth’s Past
Imagine yourself as a geological detective, embarking on a thrilling journey to unravel the secrets of Earth’s ancient history. Your trusty tool in this adventure is radiometric dating, a technique that unlocks the time capsule hidden within sedimentary rocks.
The Art of Radioactive Decay
At the heart of radiometric dating lies the fascinating process of radioactive decay. Certain elements, known as radioactive isotopes, have atoms that are unstable and prone to disintegration. When these isotopes decay, they emit particles, transforming into different elements. This transformation is the key to understanding how radiometric dating works.
Parent and Daughter Isotopes: A Family Affair
Parent isotopes are the radioactive isotopes that decay. Daughter isotopes are the stable isotopes that result from this decay. As time passes, parent isotopes decay at a constant rate, while daughter isotopes accumulate in the rock.
The Clock of Half-Life
Every radioactive isotope has a characteristic half-life, the time it takes for half of its atoms to decay. This half-life, measured in years or millions of years, plays a crucial role in determining the age of the rock.
Decoding the Time Capsule: Radiometric Techniques
Scientists use various radiometric techniques to determine the age of sedimentary rocks. One common technique is potassium-argon dating, which measures the ratio of potassium-40 (parent isotope) to argon-40 (daughter isotope). By comparing this ratio to the known half-life of potassium-40, geologists can determine the rock’s age.
Applications: Unraveling Earth’s Story
Radiometric dating is a powerful tool in unraveling Earth’s history. It has been used to:
- Determine the age of rock formations: Dating sedimentary rocks helps geologists construct a timeline of past events, from mountain formation to ocean cycles.
- Understand past climates: By analyzing isotopic ratios in sedimentary rocks, scientists can infer past temperatures, precipitation patterns, and atmospheric conditions.
- Correlate sedimentary sequences: Radiometric dating allows geologists to compare rocks from different locations and time periods, providing insights into basin evolution and global events.
Radiometric dating is a remarkable technique that provides us with a window into Earth’s distant past. By understanding radioactive decay and the relationships between parent and daughter isotopes, geologists can trace the age and history of sedimentary rocks, unlocking the secrets of our planet’s evolution.
Applications of Radiometric Dating in Geology: Unraveling Earth’s Past
Determining the Age of Geological Formations
Radiometric dating has revolutionized our understanding of Earth’s history by providing precise ages for rock formations. By measuring the decay of radioactive isotopes within rocks, geologists can determine how long ago the rocks formed. This has allowed us to establish a detailed geological timeline, uncovering the sequence of events shaping our planet.
Understanding Past Environments
Beyond dating, radiometric dating also provides insights into past environments. By analyzing the isotopic composition of sedimentary rocks, geologists can learn about the conditions under which they were formed. For example, measuring the ratio of oxygen isotopes can reveal changes in ancient ocean temperatures.
Correlating Sedimentary Sequences
Radiometric dating is crucial for correlating sedimentary sequences across different regions. By comparing the ages of similar rock layers in different locations, geologists can trace the extent and continuity of ancient geological events. This helps reconstruct past geography and understand the large-scale processes that have shaped Earth’s surface.
Reconstructing Earth’s Geological Timeline
Radiometric dating has been instrumental in reconstructing Earth’s geological timeline. By dating rocks from different eras, geologists have pieced together a comprehensive picture of the planet’s history, from the formation of the first continents to the present day. This timeline has provided a framework for understanding the evolution of life and the dynamic nature of our planet.