Quantifying Heat Release: A Guide To Heat Of Combustion Calculations
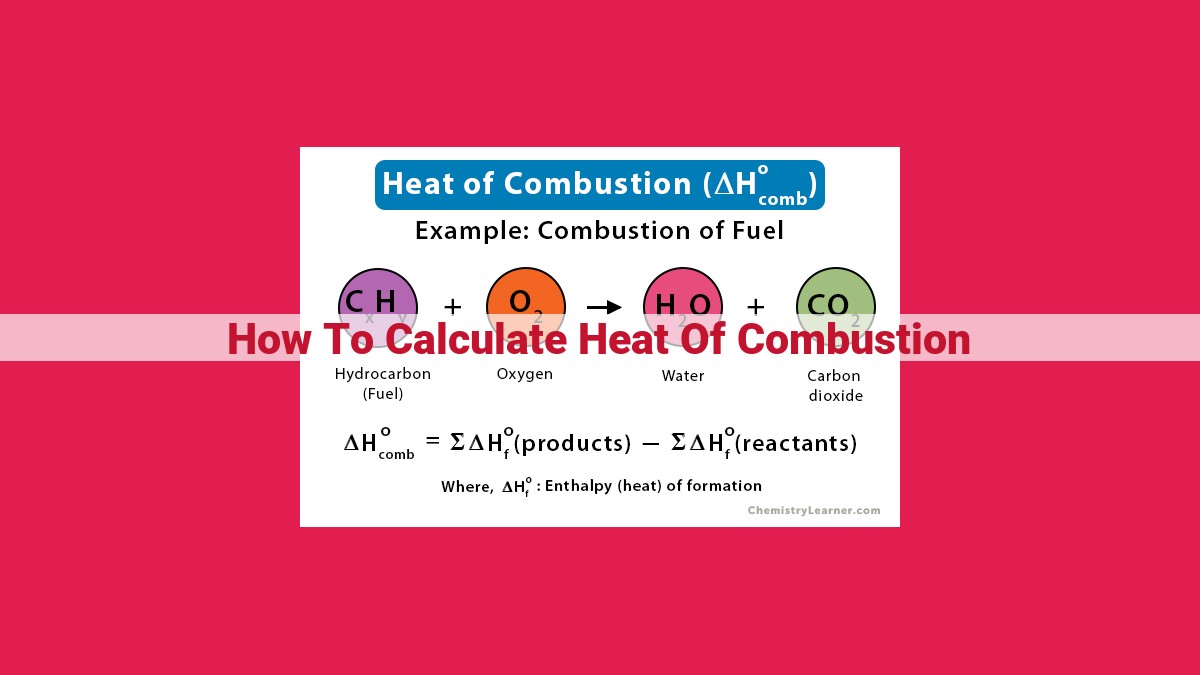
Heat of combustion is the amount of heat released when a substance undergoes a complete chemical reaction with oxygen. To calculate it, use a calorimeter to measure the temperature change of a known mass of water during combustion. Alternatively, use enthalpy of combustion data and Hess’s Law to determine the heat released based on stoichiometry and balanced equations. Factors like fuel composition and combustion conditions affect heat of combustion, which is crucial for fuel efficiency calculations, combustion system design, and environmental impact assessments.
How to Calculate Heat of Combustion: A Comprehensive Guide
Imagine you’re sitting around a cozy campfire, the flames dancing and crackling, radiating warmth. Have you ever wondered about the scientific process behind that comforting heat? It’s all about heat of combustion, the energy released when fuel burns. Understanding this concept is crucial in various fields, from chemistry to engineering and environmental science.
What is Heat of Combustion?
Heat of combustion is the quantity of heat released when a specific amount of a substance undergoes complete combustion with oxygen. It’s a measure of how much energy is stored in a fuel, and it’s often expressed in kilojoules per mole (kJ/mol).
Why is Heat of Combustion Important?
Knowing the heat of combustion is essential for:
- Fuel efficiency calculations: It helps us determine how much energy we get from different fuels, allowing us to maximize their efficiency.
- Combustion system design: Engineers use heat of combustion to design efficient furnaces, boilers, and engines that utilize fuel effectively.
- Environmental impact assessments: It aids in understanding the carbon footprint of fuels and their impact on climate change.
By calculating heat of combustion, we gain insights into the energy potential of fuels and can make informed decisions about their use and impact on our environment.
Key Concepts and Relationships in Heat of Combustion
Understanding the heat of combustion is essential in various scientific fields. To calculate it precisely, it’s crucial to grasp the following key concepts:
Calorimeter: The Heat-Measuring Device
A calorimeter plays a pivotal role in measuring heat of combustion. It’s a device designed to capture the **heat** released during combustion. By measuring the temperature change within the calorimeter, scientists can determine the amount of heat released by the burning fuel.
Calorific Value: Fuel’s Energy Potential
Calorific value refers to the energy content per unit mass or volume of a fuel. It’s directly related to the heat of combustion. A higher calorific value indicates that the fuel releases more heat upon combustion. This property is crucial for determining a fuel’s efficiency and suitability for various applications.
Enthalpy of Combustion: Thermodynamic Perspective
Enthalpy of combustion is the enthalpy change accompanying the complete combustion of a substance. It represents the total heat released or absorbed during the reaction. Negative enthalpy changes indicate exothermic reactions, where heat is released. Conversely, positive enthalpy changes denote endothermic reactions, where heat is absorbed.
Stoichiometry and Balanced Equations: Precision in Calculations
Stoichiometry and balanced chemical equations are fundamental for accurately calculating heat of combustion. Stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions, while balanced equations ensure that the number of atoms of each element remains the same on both sides of the equation. These principles help determine the exact amount of reactants and products involved, which is crucial for precise heat of combustion calculations.
Calculating Heat of Combustion: Unveiling the Energy Potential of Fuels
A. Using a Calorimeter: Witnessing Combustion’s Exothermic Dance
Step into the realm of calorimeters, the scientific instruments that dance alongside combustion, capturing its fiery symphony. Inside this sealed chamber, precisely known as a bomb calorimeter, a carefully weighed sample of fuel ignites, unleashing a surge of heat energy.
This energy is meticulously collected by the calorimeter’s water bath, causing its temperature to rise. The temperature change, measured with utmost precision, holds the key to determining the heat of combustion. With simple calculations, the amount of heat released per unit mass of fuel is revealed.
B. Using Enthalpy of Combustion Data: Harnessing Tables and Hess’s Law
Alternatively, if an enthalpy of combustion value is readily available from tables or databases, the journey to calculating heat of combustion becomes even more direct. This value represents the heat released when one mole of the fuel combusts completely.
Harnessing the power of Hess’s Law, we can piece together the heat of combustion for complex reactions by manipulating simpler reactions. This technique requires a deep understanding of stoichiometry and balanced chemical equations to ensure that every atom is accounted for.
Unraveling the Secrets: Factors that Shape Heat of Combustion
The heat of combustion is not a static entity; it dances to the tune of several influential factors:
- Fuel Composition: The molecular structure and elemental composition of the fuel dictate its energy density. Fuels with higher carbon content generally possess higher heats of combustion.
- Impurities: Contaminants or additives can alter the fuel’s energy content, potentially reducing its heat of combustion.
- Combustion Conditions: Temperature, pressure, and oxygen availability significantly impact combustion’s efficiency, thereby affecting the heat of combustion.
Embracing the Fire: Applications of Heat of Combustion
The knowledge of heat of combustion empowers us in numerous practical applications:
- Fuel Efficiency Calculations: Engineers optimize engines and fuel systems by accurately predicting the energy output of fuels based on their heat of combustion.
- Combustion System Design: Industries design efficient combustion systems, such as furnaces and boilers, by considering the heat of combustion of the intended fuels.
- Environmental Impact Assessments: Researchers assess the environmental impact of combustion processes, including greenhouse gas emissions and air pollution, by evaluating the heat of combustion.
Calculating heat of combustion is a fundamental skill in various scientific disciplines, unlocking the secrets of fuel performance and combustion processes. By mastering these techniques, we gain a deeper appreciation for the energy that fuels our world and empowers us to harness it responsibly.
Factors Affecting Heat of Combustion
Every fuel’s heat of combustion is unique and dynamic, influenced by a medley of factors that dance together to shape its energetic release.
The Composition of the Fuel: The Symphony of Elements
The very essence of a fuel, its composition, is a maestro orchestrating the heat of combustion. Fuels rich in carbon and hydrogen, like propane and gasoline, leap into combustion with gusto, releasing a symphony of heat. On the other hand, fuels with higher oxygen content, like ethanol or methanol, have a more subdued performance, releasing less heat per unit mass.
The Presence of Impurities: Unwelcome Guests at the Combustion Ball
Impurities, like unwelcome guests at a grand ball, can disrupt the harmonious dance of combustion. Inert materials like ash and moisture lurk within fuels, diluting their combustible content and dampening the heat they release.
The Conditions of Combustion: A Delicate Balance
The conditions under which combustion occurs are as crucial as the fuel itself. When the flames dance at higher temperatures, the bonds within the fuel molecules break more readily, unleashing a more energetic heat release. Pressure, too, plays a subtle role, influencing the availability of oxygen and thus affecting the heat of combustion.
In the pages of chemistry and engineering, the understanding of these factors is paramount. They empower scientists and engineers to design fuel-efficient systems, optimize combustion processes, and minimize environmental impact. From the roar of a rocket engine to the warmth of a cozy fireplace, the heat of combustion continues to captivate and inspire us all.
Calculating Heat of Combustion: A Journey into Energy Measurements
Applications of Heat of Combustion: Empowering Science and Engineering
The concept of heat of combustion extends beyond theoretical calculations and finds practical applications in various fields, revolutionizing industries and shaping environmental strategies.
Fuel Efficiency Calculations: Optimizing Energy Usage
Understanding heat of combustion empowers engineers to optimize fuel efficiency in engines, heating systems, and vehicles. By calculating the heat released during fuel combustion, designers can fine-tune engine performance, reduce fuel consumption, and minimize emissions.
Design of Combustion Systems: Harnessing Energy Efficiently
In the realm of energy production, heat of combustion plays a pivotal role in designing efficient combustion systems. Power plants and industrial boilers rely on the precise calculation of heat released to engineer systems that maximize fuel utilization and minimize energy waste.
Environmental Impact Assessments: Shaping Sustainable Practices
The heat of combustion serves as a key metric in environmental impact assessments. By quantifying the energy released from combustion processes, scientists can assess the potential emissions of greenhouse gases, particulate matter, and other pollutants. This information empowers policymakers to develop regulations and promote sustainable practices that protect our planet.
In conclusion, the calculation of heat of combustion is not just a scientific exercise but a tool that drives innovation and empowers decision-making in critical fields. From optimizing fuel efficiency to designing efficient combustion systems and evaluating environmental impacts, heat of combustion remains an indispensable concept at the heart of scientific advancements.