Quantifying Bond Polarity: Unveiling Percent Ionic Character
To find the percent ionic character, determine the electronegativity difference between bonded atoms using the Pauling scale. The larger the difference, the more polar the bond. Use the formula: %Ionic character = (1 – e^(-ΔEN^2)) x 100. Convert the result to a percentage. A higher percent ionic character indicates a more ionic bond, while a lower percentage indicates a more covalent bond. Understanding percent ionic character aids in comprehending bond nature and predicting chemical properties.
Ionic Character: Unveiling the Nature of Chemical Bonds
In the realm of chemistry, understanding the ionic character of a bond is crucial for deciphering its nature and properties. Ionic character represents the extent to which a bond exhibits the characteristics of an ionic bond, where one atom donates an electron to another, creating oppositely charged ions. This article will embark on a journey to guide you in understanding and calculating the percent ionic character of a bond.
As we delve into the world of chemical bonding, we encounter the concept of electronegativity, which measures an atom’s ability to attract electrons towards itself. The Pauling electronegativity scale provides a quantitative measure of electronegativity, helping us gauge how strongly an atom “pulls” on the shared electrons in a bond.
The difference in electronegativity between bonded atoms creates a bond polarity, resulting in a partial separation of charge. This charge separation is quantified by the percent ionic character, which indicates the degree to which the bond resembles an ionic bond.
Calculating the percent ionic character involves a straightforward formula:
% Ionic Character = 100 * (1 - e^(-ΔEN/4))
where ΔEN is the electronegativity difference between the bonded atoms. This formula reflects the exponential relationship between electronegativity difference and ionic character.
To find the percent ionic character of a bond, you embark on the following steps:
- Determine the electronegativity of each atom involved in the bond using the Pauling electronegativity scale.
- Calculate the electronegativity difference between the atoms.
- Substitute the electronegativity difference into the formula and calculate the percent ionic character.
Understanding the percent ionic character empowers us to analyze the nature of chemical bonds. Bonds with a higher percent ionic character exhibit stronger electrostatic interactions and are more likely to form ionic crystals. Conversely, bonds with a lower percent ionic character are more covalent, characterized by shared electron pairs.
By grasping the concept of percent ionic character, you gain a deeper understanding of the forces that govern chemical bonds. This knowledge empowers you to predict bond properties, analyze molecular structures, and unravel the intricate world of chemical interactions.
Electronegativity: The Key to Understanding Bond Polarity
Electronegativity is a fundamental concept in chemistry, describing an atom’s ability to attract electrons towards itself. It plays a crucial role in understanding the polarity of chemical bonds and the chemical properties of compounds.
The Pauling electronegativity scale provides a quantitative measure of an atom’s electronegativity. Higher numbers indicate greater electronegativity. For example, fluorine has the highest electronegativity (4.0), while cesium has the lowest (0.7).
Electronegativity differences between atoms in a bond determine its polarity. When two atoms with different electronegativities bond, the more electronegative atom attracts electrons more strongly, creating a partial negative charge. The other atom becomes partially positive. This partial charge separation leads to the formation of a polar bond.
The extent of polarity is directly related to the difference in electronegativity. Larger differences result in more polar bonds, while smaller differences indicate less polar or even nonpolar bonds.
Understanding electronegativity is essential for comprehending the nature of chemical bonds and the behavior of molecules. It is the foundation for further exploration of chemical concepts, such as percent ionic character, which quantifies the degree of ionicity in a covalent bond.
Concept: Pauling Electronegativity
Electronegativity is a fundamental property that measures an atom’s ability to attract electrons towards itself. It plays a pivotal role in determining bond polarity and the nature of chemical bonds. The Pauling electronegativity scale is a widely used measure of electronegativity, assigning values to different elements based on their ability to attract electrons.
When two atoms with different electronegativities form a bond, the more electronegative atom exerts a stronger pull on the shared electrons. This unequal sharing of electrons results in partial charge separation, creating a polar bond. The difference in electronegativity between the bonded atoms determines the degree of polarity. The larger the electronegativity difference, the more polar the bond.
For instance, consider a bond between sodium (Na) and chlorine (Cl). Chlorine has a higher electronegativity than sodium. As a result, the shared electrons in the bond are pulled closer to the chlorine atom, creating a polar bond with a partial negative charge on the chlorine and a partial positive charge on the sodium.
The electronegativity difference also influences the bond’s strength. Bonds with large electronegativity differences tend to be more ionic, while bonds with small differences are more covalent. Ionic bonds result from the complete transfer of electrons from one atom to another, creating charged ions. Covalent bonds, on the other hand, involve the sharing of electrons between atoms.
Understanding electronegativity is crucial for comprehending the nature of chemical bonds and the properties of different compounds. It provides valuable insights into the polarity, strength, and overall characteristics of chemical interactions.
Percent Ionic Character: Understanding the Significance
Ionic character plays a crucial role in understanding the nature of chemical bonds. It helps us determine the extent to which a bond resembles an ionic bond, characterized by a complete transfer of electrons from one atom to another. This article aims to guide you through the concept of percent ionic character, empowering you to analyze and interpret bond characteristics.
Formula and Interpretation:
- The percent ionic character of a bond represents the percentage of its character that can be attributed to ionic bonding. It is calculated using the following formula:
Percent Ionic Character = 100 x (1 - e^(-ΔEN^2/4))
- where ΔEN is the difference in electronegativity between the two bonded atoms.
Relationship with Electronegativity Difference:
Electronegativity, a measure of an atom’s ability to attract electrons, is central to understanding percent ionic character. When there is a significant difference in electronegativity between two atoms, electrons tend to shift towards the more electronegative atom, leading to the formation of partial charges. The greater the electronegativity difference, the higher the percent ionic character.
Determining Percent Ionic Character:
To calculate the percent ionic character of a bond:
- Determine the electronegativity (EN) of each bonded atom using the Pauling scale.
- Calculate the electronegativity difference: ΔEN = EN(more electronegative atom) – EN(less electronegative atom).
- Plug the ΔEN value into the formula.
- Convert the result to a percentage.
Procedure: Finding Percent Ionic Character:
- List the steps involved in finding the percent ionic character:
- Determine electronegativity of each atom.
- Calculate difference in electronegativity.
- Plug values into the formula.
- Convert the result to percentage.
Finding Percent Ionic Character: A Step-by-Step Guide
In the realm of chemistry, understanding the ionic character of chemical bonds is crucial for unraveling their nature and properties. This article will guide you through the fascinating journey of determining the percent ionic character of a bond using the electronegativity scale. Buckle up and let’s dive into this exciting exploration!
Electronegativity: The Key to Bond Polarity
Electronegativity measures how strongly an atom attracts electrons towards itself. The Pauling electronegativity scale provides a numerical value for each element, quantifying their electronegative strength. When atoms with different electronegativities combine, they create bond polarity. The more electronegative atom pulls electrons closer to itself, creating a partial negative charge. The less electronegative atom, in turn, becomes partially positive.
The Percent Ionic Character Formula
The percent ionic character of a bond quantifies the extent to which it exhibits ionic character. It’s calculated using the following simple formula:
Percent Ionic Character = (1 - e^(-0.25ΔEN²)) * 100%
where ΔEN is the difference in electronegativity between the two atoms.
Step-by-Step Procedure
Follow these straightforward steps to determine the percent ionic character:
-
Determine electronegativity: Use a reference table or the Pauling electronegativity scale to find the electronegativity values for each atom.
-
Calculate difference in electronegativity: Subtract the electronegativity of the less electronegative atom from that of the more electronegative atom.
-
Plug values into the formula: Substitute the electronegativity difference into the percent ionic character formula.
-
Convert to percentage: Multiply the result by 100% to express the ionic character as a percentage.
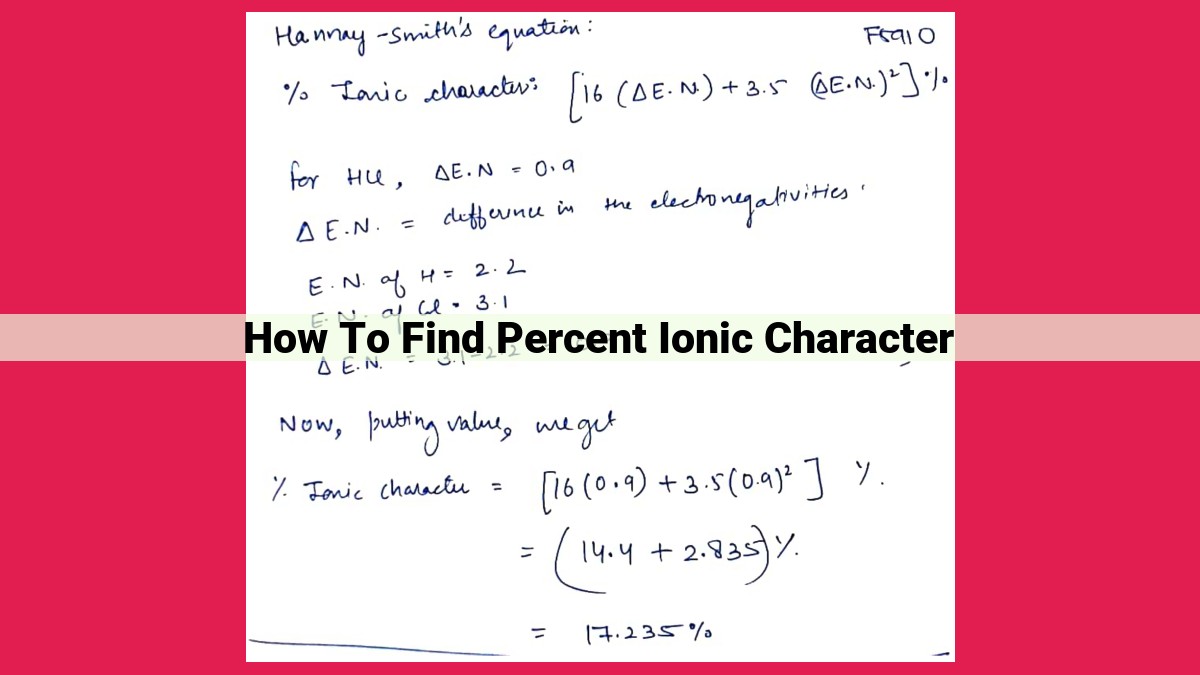
Example: NaCl Bond
Let’s calculate the percent ionic character of the bond between sodium (Na) and chlorine (Cl).
- Electronegativity: Na = 0.9, Cl = 3.0
- Difference in electronegativity (ΔEN): 3.0 – 0.9 = 2.1
Plugging into the formula:
Percent Ionic Character = (1 - e^(-0.25(2.1)²)) * 100%
= (1 - e^(-0.25(4.41))) * 100%
= (1 - 0.060) * 100%
= 93.95%
Therefore, the NaCl bond has a high percent ionic character, indicating its strong ionic nature.
Understanding percent ionic character empowers us to discern the true nature of chemical bonds. By considering the electronegativity differences between atoms, we can predict the polarity and ionic character of the bond they form. Embrace this knowledge as a superpower in your pursuit of chemical understanding!
Uncover the Secrets of Ionic Bonds: A Guide to Finding Percent Ionic Character
Ionic bonds play a crucial role in understanding chemical reactions and properties. They form when one atom transfers electrons to another, resulting in positively and negatively charged ions. Determining the ionic character of a bond helps us understand its polarity and strength.
Electronegativity: The Key Player
Electronegativity measures an atom’s ability to attract electrons. The higher the electronegativity, the stronger its pull. Electronegativity differences between atoms determine the polarity of a bond.
Pauling Electronegativity:
The Pauling electronegativity scale quantifies electronegativity. By comparing the electronegativity values of the bonded atoms, we can determine the partial charge separation caused by the unequal electron sharing.
Percent Ionic Character:
To quantify the ionic character of a bond, we use the following formula:
% Ionic Character = (1 - e^(-0.25 * ΔEN^2)) * 100%
where ΔEN is the absolute difference in electronegativity between the bonded atoms.
Procedure: Finding Percent Ionic Character
To find the percent ionic character, follow these steps:
- Determine the electronegativity of each atom using the Pauling electronegativity scale.
- Subtract the electronegativities to find ΔEN.
- Plug the value of ΔEN into the formula.
- Convert the result to a percentage.
Example:
Let’s calculate the percent ionic character of the bond between sodium (Na) and chlorine (Cl). Na has an electronegativity of 0.9, while Cl has an electronegativity of 3.0.
ΔEN = 3.0 - 0.9 = 2.1
% Ionic Character = (1 - e^(-0.25 * (2.1)^2)) * 100%
= (1 - e^(-0.25 * 4.41)) * 100%
= 70.2% (approximately)
This relatively high percent ionic character indicates that the Na-Cl bond is predominantly ionic.