Quantifying The Atomic Composition Of Cells: A Comprehensive Guide
How Many Atoms Are in a Cell?
Determining the number of atoms in a cell requires understanding Avogadro’s Number, the mole, molar mass, atomic mass unit, cell volume, atomic arrangement, molar weight, and molecular weight. The atomic arrangement within a crystal structure dictates the number of atoms per unit cell. By determining the molar mass of a cell’s constituents and its volume, the total number of atoms can be estimated. This involves considering the organization of cells into tissues, organs, and organisms, and applying concepts such as density to calculate the number of cells present.
Units of Measurement: Understanding the Building Blocks of Matter
Avogadro’s Number: A Key to Counting Atoms
Imagine yourself standing in a vast warehouse filled with tiny marbles, each representing an atom. To count these marbles, you would need to know their number. Avogadro’s Number provides that measure, representing the colossal number of particles present in one mole of a substance. This constant, approximately 6.022 x 10^23, allows scientists to quantify the number of atoms in a given sample.
The Mole: A Standardization for Matter
The mole is the fundamental unit of measurement for the amount of substance. It is defined as the amount of a substance that contains exactly Avogadro’s Number of particles, which can be atoms, molecules, or ions. The mole serves as a standardized way to express the quantity of matter in chemical reactions and other scientific calculations.
Molar Mass: Determining the Mass of Atoms
Every atom has a specific molar mass, which is the mass of one mole of that atom. The molar mass is expressed in grams per mole (g/mol) and provides a convenient way to determine the mass of a known number of atoms. For example, one mole of carbon has a molar mass of 12.01 g/mol, indicating that 1 mole of carbon atoms weighs 12.01 grams.
The Gas Constant: Linking Pressure, Volume, and Temperature
The gas constant is a fundamental physical constant that relates the pressure, volume, and temperature of a gas. It is represented by the symbol R and has a value of 0.0821 (L·atm)/(mol·K). The gas constant aids in calculations involving gas properties and provides insights into the behavior of gases under various conditions.
Atomic Mass Unit: The Building Block of Matter
In the microscopic realm of atoms, the atomic mass unit (amu) plays a pivotal role in understanding the composition and properties of everything that exists. The amu, also known as the Dalton, provides a standard measure for the mass of atoms.
Imagine a universe where every atom has a tiny scale attached to its nucleus. The amu is the unit of measurement used on this scale, where each hydrogen atom weighs exactly 1 amu. This means that every other atom in the periodic table has a mass expressed as a multiple of the hydrogen atom’s mass.
Atomic Weight and Molar Mass: Key Concepts for Cell Biology
-
Atomic Weight: The atomic weight of an element is the average mass of its atoms, taking into account the naturally occurring isotopes of that element. For example, carbon has two stable isotopes with masses of 12 amu and 13 amu, respectively. Its atomic weight is the weighted average of these masses, resulting in an atomic weight of approximately 12.01 amu.
-
Molar Mass: The molar mass of a compound or element is the mass of one mole of that substance. A mole is a colossal number (approximately 6.022 × 10^23) that represents a specific quantity of any substance. The molar mass of a compound is the sum of the atomic weights of all the atoms in its molecular formula.
For example, the molar mass of carbon dioxide (CO2) is 44.01 g/mol, calculated as the sum of the atomic weights of one carbon atom (12.01 amu) and two oxygen atoms (16.00 amu each). Understanding atomic mass units, atomic weight, and molar mass is essential for cell biologists, as these concepts provide the foundation for quantifying and understanding the chemical composition of cells.
Cell Volume: Unraveling the Microscopic Building Blocks
Understanding the volume of a cell is crucial to grasping the vast number of atoms that reside within its confines. Just as a towering skyscraper is composed of countless bricks, a cell is an intricate structure assembled from equally numerous atoms.
The Unit Cell: A Microscopic Foundation
Picture a tiny brick, the fundamental building block of a crystal. This microscopic marvel, known as a unit cell, represents the smallest repeating unit of a crystal’s structure. The arrangement of these unit cells, like tiny tiles in a mosaic, determines the overall volume of the crystal.
Crystal Structure: A Lattice of Atoms
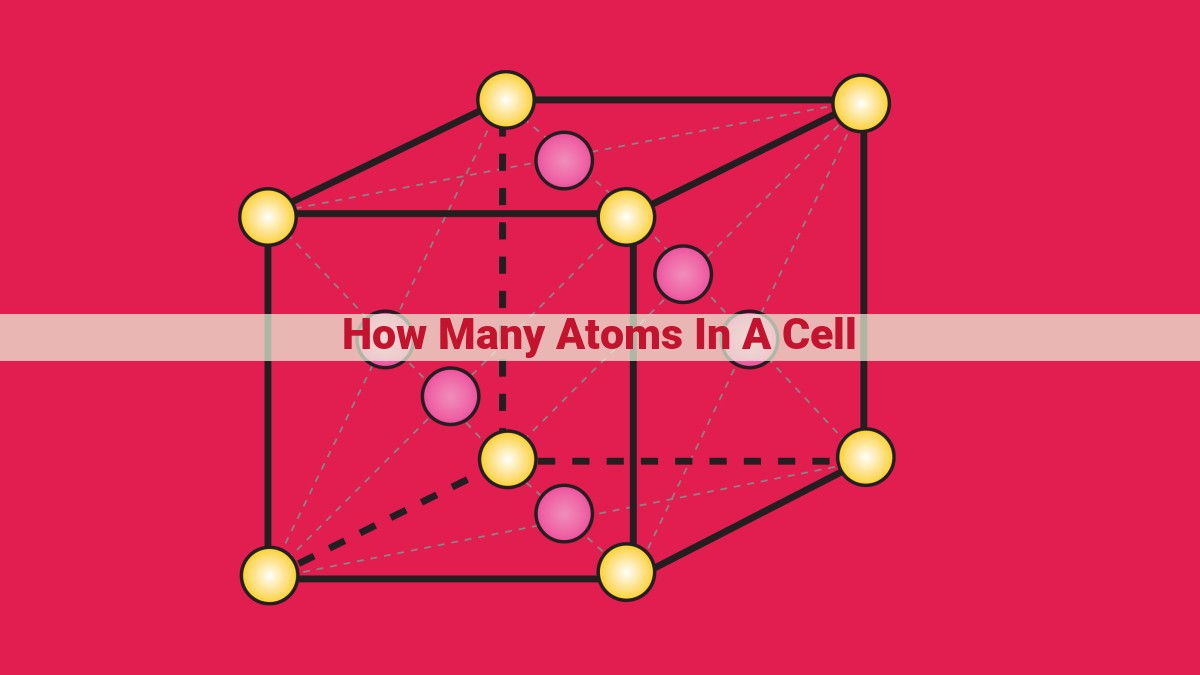
Cells, too, possess a defined crystal structure that dictates their volume. These structures can vary widely, from simple cubic arrangements to complex hexagonal lattices. **The arrangement of atoms within the unit cell influences the overall cell volume. For instance, a cubic unit cell typically contains eight atoms at its corners, whereas a face-centered cubic unit cell boasts 12 atoms, four on each face and four in the center.
By understanding the unit cell and crystal structure of a cell, we can make inferences about its overall volume. This knowledge lays the groundwork for estimating the staggering number of atoms that reside within each microscopic cell.
Additional Notes for Optimization:
- Keywords: Cell volume, unit cell, crystal structure, crystal lattice, atomic arrangement.
- Headings: Use subheadings to break up the text and make it more readable.
- Formatting: Use boldface, italics, and underlining to highlight important terms and concepts.
- Paragraphs: Keep paragraphs concise and easy to digest.
- Storytelling: Use a conversational tone and relatable examples to engage readers.
Number of Atoms per Cell: A Tale of Crystal Structure and Atomic Arrangement
In the realm of atoms and cells, the number of atoms packed within a cell is dictated by a dance between two intricate factors: atomic arrangement and crystal structure. These elements play a pivotal role in determining the number of atoms that occupy a single unit cell.
A unit cell is the smallest repeating unit of a crystal. It’s the fundamental building block that tiles together to form the larger crystal structure. The arrangement of atoms within the unit cell determines the crystal’s overall symmetry and properties.
Crystal structure refers to the regular, three-dimensional arrangement of atoms in a crystal. Different crystal structures, such as cubic, hexagonal, and tetragonal, exhibit distinct patterns of atomic packing. This packing dictates how many atoms can be accommodated within a given unit cell.
For example, a cubic crystal structure, with its symmetrical arrangement of atoms, can house more atoms per unit cell compared to a hexagonal crystal structure. This is because the cubic structure allows for a tighter packing of atoms, maximizing the number of atomic residents within the cell.
The atomic arrangement within the unit cell also influences the number of atoms. Different atomic arrangements, such as face-centered cubic (fcc) and body-centered cubic (bcc), result in variations in the number of atoms.
In an fcc unit cell, atoms are positioned at each corner and the center of each face. This arrangement accommodates a total of 14 atoms per unit cell. In contrast, a bcc unit cell has atoms at each corner and the center of the cell, resulting in 8 atoms per unit cell.
By understanding the interplay between atomic arrangement and crystal structure, scientists can determine the number of atoms that reside within a unit cell, providing valuable insights into the composition and properties of materials and substances.
Defining Molar Mass and Its Importance in Cell Counting
In the realm of understanding the microscopic world of cells, determining the number of atoms within their structure is a fundamental aspect. To embark on this journey, we must first delve into the concept of molar mass, a crucial unit of measurement in chemistry.
Molar mass, measured in grams per mole (g/mol), is defined as the mass of a substance that contains exactly one mole of its atoms, molecules, or ions. The mole, represented by the symbol “mol,” is itself a unit that represents an enormous quantity of entities, specifically 6.022 x 1023. This immense number, known as Avogadro’s number, is pivotal in relating atomic and molecular scales to macroscopic measurements.
Molar Mass and Chemical Formulas
The molar mass of a compound is directly related to its chemical formula. A chemical formula is a succinct representation of the elemental composition of a molecule, indicating the type and number of each atom present. For example, the chemical formula for carbon dioxide is “CO2,” indicating that one molecule of carbon dioxide contains one carbon atom and two oxygen atoms.
The molar mass of a compound is calculated by summing the atomic masses of all its constituent atoms, taking into account the number of atoms of each element. For instance, the molar mass of carbon dioxide is approximately 44 g/mol, obtained by adding the atomic mass of carbon (12 g/mol) to the atomic mass of two oxygen atoms (16 g/mol x 2).
Relevance to Cell Counting
Determining the molar mass of a cell is crucial for cell counting, as it allows us to relate the mass of a cell sample to the number of cells it contains. By combining cell volume, density, and molar mass, we can derive an accurate estimate of the number of atoms within an individual cell. This knowledge is essential in various scientific disciplines, including cell biology, biochemistry, and medicine, where understanding the atomic composition of cells is paramount.
Molecular Weight: The Building Blocks of Cells
Understanding the Foundation
Every cell in our bodies, from the tiny skin cells to the complex neurons in our brains, is composed of an intricate network of atoms. But just how many atoms make up a single cell? To answer this question, we need to delve into the realm of molecular weight.
From Atoms to Molecules
Atoms are the fundamental building blocks of all matter, including cells. Each atom has a specific mass, measured in atomic mass units (amu). However, atoms rarely exist in isolation. They combine to form molecules, which themselves have a molecular weight.
Molecular Weight: Adding It Up
The molecular weight of a molecule is simply the sum of the atomic masses of all the atoms that make it up. For example, a molecule of water (H2O) has two hydrogen atoms and one oxygen atom. The atomic mass of hydrogen is 1 amu, and the atomic mass of oxygen is 16 amu. So, the molecular weight of water is 18 amu (2 x 1 amu + 1 x 16 amu).
Molar Mass and Avogadro’s Number
Molar mass is a closely related concept that expresses the mass of one mole of a substance. A mole is a vast number (6.022 x 10^23) of atoms, molecules, or other particles. The molar mass of a substance is numerically equal to its molecular weight, but the units are grams per mole (g/mol) instead of amu.
Relevance to Cell Counting
Understanding molecular weight is crucial for counting cells. By knowing the molecular weight of a cell’s component molecules, scientists can determine the number of atoms in a single cell. This information provides valuable insights into cell composition and function.
Number of Cells: The Building Blocks of Life
Organization of Cells
Cells, the fundamental units of life, are not isolated entities. They form intricate networks, cooperating and communicating to create complex organisms. The smallest level of organization is tissue, a group of similar cells that perform a specific function. Tissues, in turn, combine to form organs, which have more specialized roles. Finally, organs work together within organisms, the complete living entity.
Cell Counting Techniques
Determining the number of cells in an organism or tissue is crucial for understanding its growth, development, and health. One common technique is cell counting using a hemocytometer. This device consists of a gridded chamber that allows researchers to count the number of cells in a diluted sample under a microscope.
Density and Cell Number
The density of a substance is the ratio of its mass to its volume. In the context of cells, density can be used to estimate the number of cells in a given volume. By measuring the mass and volume of a cell suspension, researchers can calculate its density and use this information to determine the number of cells present.
Significance of Cell Number
The number of cells in an organism or tissue provides valuable insights into its overall health and function. For example, an abnormally high number of cells in a tissue can indicate the presence of a tumor, while a decrease in cell number could suggest cell death or tissue damage. Understanding the number of cells is crucial for diagnosing diseases, monitoring treatment outcomes, and studying cellular processes.
The number of cells in an organism or tissue is a critical measure of its health and complexity. By understanding the organization of cells into tissues, organs, and organisms, and employing techniques such as cell counting and density measurements, researchers can gain valuable insights into the fundamental building blocks of life.
How Many Atoms Are in a Cell: A Comprehensive Guide
In the world of microscopic marvels that make up life, understanding the number of atoms within a cell holds immense significance. This blog post will embark on a captivating journey to unveil the secrets of this intricate calculation.
Units of Measurement: The Foundation
To grasp the number of atoms in a cell, we must lay a solid foundation of units of measurement. One of the most crucial is Avogadro’s Number, which represents the staggering number of atoms in exactly 12 grams of pure carbon-12. This number, approximately 6.022 x 10^23, forms the basis for defining the mole, which is the amount of substance containing that many atoms.
Furthermore, each element has its own unique molar mass, which is the mass of one mole of atoms of that element. This mass is expressed in grams per mole and is calculated by summing the masses of its constituent protons, neutrons, and electrons.
Cell Volume: The Building Block
The volume of a cell is another essential factor in determining the number of atoms it contains. A cell’s volume can be estimated using various techniques, such as microscopy or flow cytometry. Understanding the cell’s volume aids in calculating the number of atoms per unit of its inner space.
Number of Atoms per Cell: Unveiling the Mystery
The arrangement of atoms within a cell is governed by the atomic structure and crystal structure of the materials it comprises. These structures dictate the packing density of atoms within the cell and, consequently, the number of atoms per unit cell.
Mass and Density: Estimation and Quantification
Mass and density play crucial roles in cell counting. Mass, measured in grams, represents the total weight of a cell, while density, expressed in grams per cubic centimeter (g/cm³), indicates the compactness of the cell’s structure. By combining these measurements, scientists can estimate the number of cells within a specific volume.