Exploring The Multifaceted Role Of “Q” In Physics: Electric Charge, Heat, Mass, Momentum, And More
In physics, the letter “Q” is commonly used to represent various quantities. It can denote electric charge (in electromagnetism), heat (in thermodynamics), mass (in mechanics), momentum (in classical mechanics and quantum mechanics), the reaction quotient (in chemical kinetics), and thermal energy (in thermodynamics).
Electric Charge: The Heart of Electrical Phenomena
Prepare yourself for a captivating journey into the fascinating world of electricity, where electric charge stands as the enigmatic force behind all its wonders.
Imagine a universe brimming with tiny particles, each carrying an invisible property called charge. Like magnets, these particles possess either a positive or negative charge, attracting or repelling each other with an invisible force. This invisible force, known as the electromagnetic force, is responsible for the mesmerizing displays of electricity we witness daily.
To quantify this enigmatic property, scientists have coined the symbol Q to represent electric charge. It’s a measure of the amount of charge a particle possesses, whether positive or negative. When particles with opposite charges come together, their charges cancel each other out, creating a neutral state. However, when particles with like charges encounter each other, their mutual attraction or repulsion amplifies, leading to the fascinating phenomena we associate with electricity.
This interplay of electric charges manifests itself in a myriad of ways. From the crackling of lightning bolts to the smooth flow of electric current in our homes, electric charge orchestrates a symphony of phenomena that shape our technological world. Join us as we delve deeper into the captivating world of electricity, where charge reigns supreme.
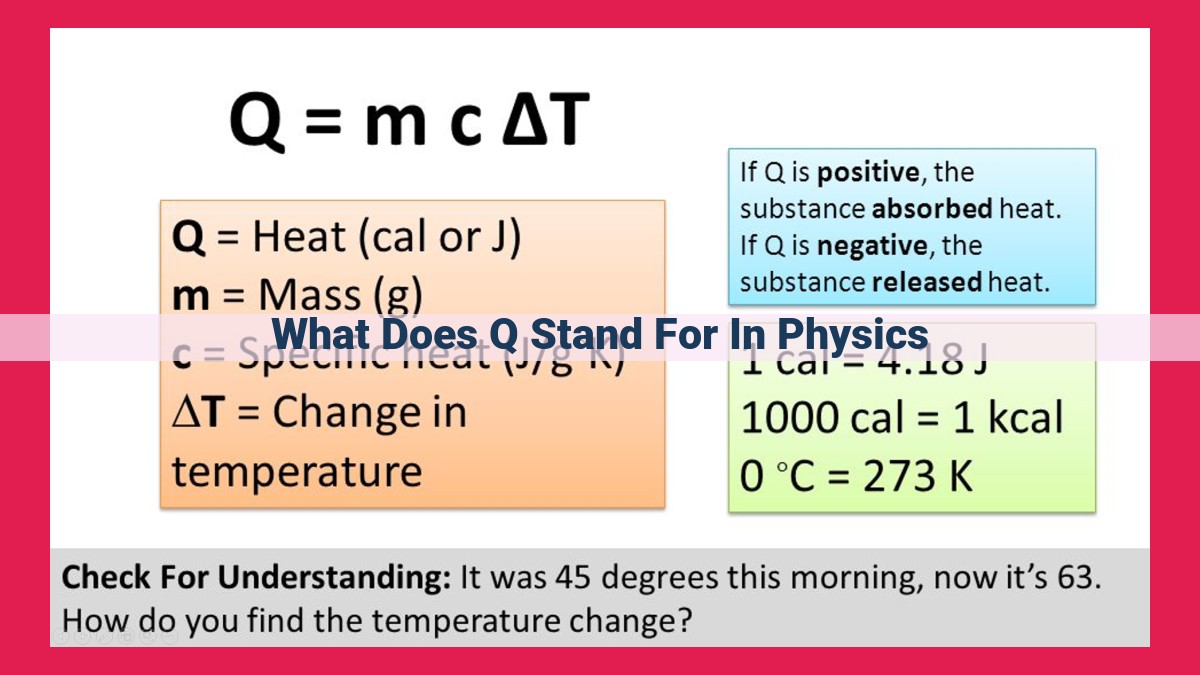
Heat: The Flow of Thermal Energy
- Explain the nature of heat as the transfer of thermal energy, using Q to quantify it.
Heat: The Flow of Thermal Energy
Imagine the cozy warmth of a crackling fire on a winter’s night. Or the refreshing coolness of a summer breeze on a sweltering afternoon. These sensations are all manifestations of heat, a fundamental concept in physics.
Heat is not a substance or a property of matter but rather a flow of thermal energy. It represents the transfer of energy from one system to another due to a temperature difference. The symbol Q is used to quantify heat, and its unit is the joule (J).
In everyday language, we often equate heat with temperature. However, these are two distinct concepts. Temperature measures the average kinetic energy of the particles within a system, while heat quantifies the total energy exchange. For instance, a cup of hot coffee has a higher temperature than a cup of cold coffee, but the hot coffee may have transferred more heat to your hand than the cold coffee.
Heat can be transferred in three primary ways:
- Conduction: Heat flows through direct contact between objects, such as when you place your hand on a warm stovetop.
- Convection: Heat is carried by the movement of fluids, such as when hot air rises in a room.
- Radiation: Heat is emitted as electromagnetic waves, such as the warmth you feel from the sun.
Understanding the nature of heat is essential in fields such as thermodynamics, engineering, and meteorology. It helps us design efficient heating and cooling systems, develop new energy sources, and predict weather patterns. By harnessing the power of heat, we can create a more comfortable and sustainable world.
Mass: The Resistance to Acceleration, Represented by Q
Mass is a fundamental property of matter, measuring an object’s resistance to changes in its motion. It represents the inertia of an object, resisting any attempt to accelerate it or change its direction or speed.
In physics, mass is often represented by the symbol Q. This letter encapsulates the concept that mass is a quantitative measure of an object’s resistance to acceleration. The greater the mass, the more difficult it is to accelerate an object, as it requires more force.
A heavier object, with a greater mass, will resist acceleration more than a lighter object. For example, a car with a greater mass requires more force to accelerate than a bicycle.
The relationship between mass and acceleration is expressed by Newton’s second law of motion. This law states that the acceleration of an object is directly proportional to the force applied to it and inversely proportional to its mass.
Mathematically, this relationship can be written as:
a = F / m
where:
- a is the acceleration of the object
- F is the force applied to the object
- m is the mass of the object
This formula highlights the inverse relationship between mass and acceleration. A greater mass leads to a smaller acceleration for a given force.
Understanding mass is crucial in various areas of science and engineering, such as mechanics, astrophysics, and materials science. It helps us describe and predict the behavior of objects in motion, from the smallest particles to the grandest cosmic structures.
Momentum: A Measure of Motion
Momentum, represented as Q, is a fundamental quantity that measures the motion of an object. It captures the combined effect of an object’s mass and velocity. Physicists define momentum mathematically as:
Q = mv
Where Q is momentum, m is mass, and v is velocity.
Momentum encapsulates an object’s resistance to change in motion. An object with greater momentum will require a larger force to alter its velocity compared to an object with less momentum. Momentum is a conserved quantity, which means the total momentum of a system remains constant in the absence of external forces. This conservation principle plays a crucial role in understanding collisions and other interactions between objects.
In everyday life, we can observe the effects of momentum in a variety of scenarios. For instance, when a heavy truck collides with a smaller car, the truck’s greater momentum results in a more devastating impact. Conversely, when a person kicks a soccer ball, the force of their kick transfers momentum to the ball, propelling it forward.
Understanding momentum is essential in fields such as physics, engineering, and sports. It allows us to predict the outcomes of collisions, design efficient mechanical systems, and enhance athletic performance. Therefore, the concept of momentum serves as a fundamental tool in our exploration of the world around us.
Reaction Quotient: Delving into Chemical Equilibrium
In the realm of chemistry, the reaction quotient (Q) emerges as a pivotal concept in our quest to unravel the intricacies of chemical reactions. It unveils the dynamic interplay between reactants and products, providing a quantitative measure of the reaction’s progress.
Imagine a hypothetical chemical reaction where two molecules of reactant A combine with one molecule of reactant B to form two molecules of product C. The reaction quotient, denoted by Q, is defined as the ratio of the concentrations of the products to the concentrations of the reactants at a given instant:
Q = [C]^2 / [A]^2[B]
Understanding Q’s Significance
The reaction quotient offers a real-time snapshot of the reaction’s progress. A high Q value indicates a strong tendency for the reaction to proceed in the forward direction, forming more products. Conversely, a low Q value suggests a preference for the reverse reaction, converting products back into reactants.
Achieving Equilibrium
As the reaction progresses, Q gradually changes until it reaches a constant value known as the equilibrium constant (K). At equilibrium, the forward and reverse reactions occur at equal rates, and the concentrations of the reactants and products remain unchanged.
The equilibrium constant, K, is a unique characteristic of the reaction that depends solely on temperature. Q, on the other hand, depends on both the temperature and the initial concentrations of the reactants and products.
Q and K: Illuminating the Reaction’s Dynamics
The relationship between Q and K provides valuable insights into the reaction’s behavior:
- Q > K: The reaction will proceed in the forward direction, shifting the equilibrium towards more products.
- Q < K: The reaction will proceed in the reverse direction, favoring the formation of reactants.
- Q = K: The reaction is at equilibrium, and the concentrations of reactants and products are constant.
Harnessing Q in Practice
Armed with the knowledge of the reaction quotient, chemists can predict the direction and extent of chemical reactions. This understanding has far-reaching applications in fields such as:
- Industrial chemistry: Optimizing reaction yields and minimizing waste.
- Biochemistry: Understanding metabolic processes and enzyme activity.
- Environmental chemistry: Assessing the impact of pollutants on ecosystems.
The reaction quotient, Q, is an indispensable tool in the chemist’s arsenal, unlocking the secrets of chemical equilibrium. By delving into its significance, we gain a deeper appreciation of the dynamic nature of chemical reactions and their profound impact on the world around us.
Thermal Energy: The Energy of Motion
Imagine a bustling city, where countless individuals move and interact, each contributing to the overall energy of the system. Thermal energy is much like this urban landscape, representing the random motion of particles that constitute matter.
Every substance, from the air we breathe to the coffee we sip, contains particles that are in constant motion. This movement, known as thermal agitation, generates the internal energy of the substance. The higher the temperature, the more vigorous the particle motion, leading to greater thermal energy.
Thermal energy is often quantified by the symbol Q and is expressed in units of joules (J). It plays a crucial role in thermodynamics, the study of heat and energy transfer. By understanding thermal energy, we can unravel the complexities of everyday phenomena, such as why hot objects transfer heat to cold objects.
Thermal energy is closely related to heat, the transfer of energy from one object to another due to a temperature difference. When heat flows into a substance, the particles gain energy and move faster, increasing the thermal energy. Conversely, when heat flows out of a substance, the particles lose energy and slow down, reducing the thermal energy.
In conclusion, thermal energy encapsulates the energy of motion within matter. It is a fundamental concept in thermodynamics and plays a pivotal role in understanding heat transfer and other energy-related phenomena. By grasping the nature of thermal energy, we can unlock a deeper comprehension of our physical world.