Discover The Fundamentals Of Pure Substances: Elements Vs. Compounds
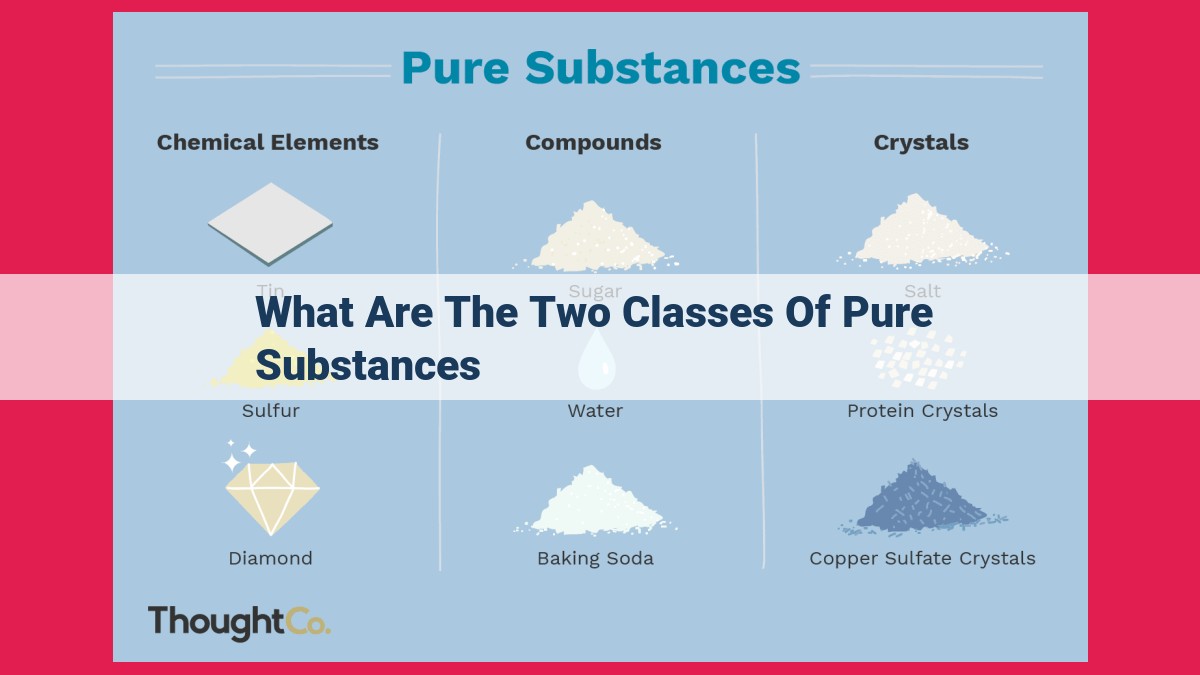
Pure substances, those with uniform composition, are classified into two groups: elements and compounds. Elements consist of atoms of only one kind, while compounds are formed when atoms of different elements chemically combine. These two classes exhibit distinct characteristics in terms of composition, structure, and properties, playing crucial roles in various fields.
Subheading: The World of Pure Substances
The World of Pure Substances: An Unseen Symphony of Matter
Within the tapestry of our existence, matter weaves a magical dance. At its purest form, this dance is performed by an exquisite cast known as pure substances. These substances, like celestial bodies in a cosmic ballet, possess an ethereal simplicity that belies their profound impact on our world.
Pure Substances: Uniting Matter’s Essence
Pure substances embody the very definition of purity. They are the building blocks of the universe, the unadulterated materials from which all else is formed. Within their molecular structure lies the blueprint for an infinite array of materials, each with its own unique properties and purpose.
Elements: The Primordial Atoms
Elements, the fundamental notes in this symphony, are the simplest form of pure substances. Each element possesses a distinct atomic number, representing the number of protons within its nucleus. Hydrogen, the lightest element, with a single proton, dances with grace and agility. Uranium, on the other hand, the heaviest known element, possesses a majestic aura with its 92 protons.
Compounds: When Elements Embark on a Molecular Adventure
Compounds, the harmonious chords that enrich the melody, arise when elements join forces. These substances are born from chemical reactions, where elements merge to create new substances, each with its own distinct personality and purpose. Water, a life-giving elixir, is a quintessential example of a compound, formed by the enchanting union of hydrogen and oxygen.
As you delve into the world of pure substances, you’ll discover a captivating narrative of matter’s transformative journey. From the dawn of elements to the alchemy of compounds, this is a story that unfolds in laboratories and textbooks, in the depths of the ocean, and in the stars that twinkle above our heads.
Dive into the World of Pure Substances: Unraveling the Secrets of Elements and Compounds
Imagine a realm where matter exists in its purest form, free from any impurities. This realm is the world of pure substances, the foundation of chemistry and the building blocks of our universe. Pure substances can be classified into two distinct categories: elements and compounds.
Elements: The Building Blocks of Nature
Elements are the fundamental units of matter. They’re pure substances composed of only one type of atom, the smallest unit of an element. Each element has a unique atomic number, which determines the number of protons in its nucleus. This atomic number defines an element’s identity and position on the Periodic Table. Atoms can exist on their own or combine with other atoms to form molecules.
Compounds: When Elements Join Forces
In contrast to elements, compounds are pure substances that result from the chemical combination of two or more different elements. Unlike molecules formed by similar atoms, compounds are formed when atoms of different elements interact and bond together. This interaction involves chemical reactions, where atoms rearrange and combine to create new substances with properties distinct from their individual constituents.
Distinguishing Elements from Compounds: Separating the Atoms from the Mix
The key to distinguishing elements from compounds lies in their atomic composition. Elements are made up of a single type of atom, while compounds are composed of two or more different types of atoms. This difference in composition results in unique properties for each class of substance. Elements have fixed compositions and cannot be broken down into simpler substances, whereas compounds can be decomposed into their constituent elements through chemical reactions.
Subheading: Elements: The Building Blocks of Nature
Elements: The Building Blocks of Nature
In the realm of pure substances, where matter unveils its pristine form, we encounter elements, the fundamental constituents of all that surrounds us. These atomic architects are the indivisible units of matter, each possessing a unique atomic number that defines its atomic identity.
Elements, like solitary stars in the celestial tapestry, shine with their own brilliance. They exist in their purest form, with each atom composed entirely of a single type of particle – the nucleus, surrounded by a cloud of electrons. This atomic simplicity grants elements remarkable stability and distinct properties.
Within the Periodic Table, a celestial chart of elements, these atomic building blocks are arranged in an elegant dance. Elements of similar electron configurations waltz together in vertical columns, known as groups, while those sharing horizontal rows, called periods, share a common number of electron shells. This systematic arrangement reveals the periodic trends in atomic properties, enabling scientists to predict the characteristics of elements based on their position in the table.
Elements, the primordial architects, hold the blueprint for the boundless diversity of matter. From the celestial brilliance of helium to the earthy abundance of iron, elements play a pivotal role in shaping the universe we inhabit. They are the foundational pillars upon which the complex tapestry of compounds and materials is woven.
Elements: The Building Blocks of Nature
In the realm of pure substances, elements stand as the fundamental constituents of matter. They are the building blocks from which all other substances are formed. Each element is characterized by a unique atomic number, representing the number of protons in its nucleus. Atoms, the basic units of elements, are composed of protons, neutrons, and electrons arranged in specific configurations.
The Periodic Table, a systematic arrangement of elements based on their atomic numbers and chemical properties, provides a comprehensive guide to the elements known to science. It categorizes elements into groups and periods, revealing patterns and relationships that shape their behavior. Through the Periodic Table, scientists can predict the properties of elements and their potential interactions with other elements.
Understanding elements is essential for unraveling the mysteries of the natural world. From the formation of stars to the chemistry of life, elements play an intricate role in shaping the universe we inhabit. By delving into the world of elements, we embark on a journey to comprehend the very essence of matter.
Understanding Elements: The Building Blocks of Nature
In the vast panorama of matter, pure substances stand as fundamental building blocks, the very essence of our existence. Among these pure substances, elements hold a unique position, their simplicity both profound and transformative.
An element is a substance that cannot be broken down into simpler substances by chemical means. It is the primordial essence of matter, endowed with only one type of atom. Atoms, the infinitesimal units of elements, are the indivisible building blocks of the universe.
The nature of elements is captured in their atomic composition. Each element possesses a unique number of protons in the nucleus of its atoms, giving it a distinct atomic number. This atomic number defines the element’s position on the legendary Periodic Table, a chemical map that organizes elements according to their properties and behaviors.
The Periodic Table serves as a guide to the breathtaking diversity of elements. From hydrogen, the lightest element with a single proton, to uranium, the heaviest naturally occurring element with 92 protons, elements adorn the Periodic Table with their unique identities.
The simplicity of elements belies their immense power. They are the fundamental building blocks from which all matter, from the air we breathe to the devices we hold in our hands, is constructed. In their pure form, elements often exhibit distinct properties that make them indispensable for various applications. For instance, gold is prized for its malleability and luster, making it a favorite for jewelry and electronics. Iron is the backbone of modern society, used in everything from construction to transportation.
As we delve into the world of pure substances, elements emerge as the cornerstone of chemistry, biology, and physics. Their fundamental nature and unique properties have shaped the course of human history and will continue to inspire scientific advancements for generations to come.
The ABCs of Chemistry: Embarking on a Journey into the World of Pure Substances
In the realm of chemistry, we encounter pure substances, the fundamental building blocks of matter that exist in two distinct forms: elements and compounds. Let’s dive into the captivating world of these fascinating substances, unraveling their unique properties and the secrets they hold.
Elements: The Unbreakable Atoms
Elements, the building blocks of nature, stand alone as pure substances composed of only one type of atom. Each atom, the fundamental unit of matter, possesses a distinct number of protons and electrons that determine its chemical behavior.
Atoms dance around the stage of existence, interacting with one another to form molecules, the tiniest units of compounds. And to help us make sense of this atomic orchestra, we have the Periodic Table, a roadmap that organizes elements based on their properties.
Compounds: When Elements Unite
Unlike elements, compounds are pure substances born from the union of two or more elements. These alliances are forged through chemical reactions, where elements exchange electrons to form chemical bonds, creating substances with new and distinct properties.
These chemical weddings result in the creation of countless compounds, each with a unique story to tell. Some compounds, like water (H2O), are essential for life, while others, like salt (NaCl), enhance our culinary experiences.
Comparing Elements and Compounds
To distinguish between elements and compounds, we look to their atomic composition. Elements, as we’ve learned, are made up of only one type of atom. Compounds, on the other hand, are a combination of two or more elements, their atoms arranged in a specific ratio.
The properties of compounds are a testament to the power of their atomic alliances. By rearranging and combining elements, compounds acquire their own unique characteristics, distinct from the elements that gave them birth. These properties are what make compounds indispensable in fields like medicine, engineering, and materials science.
In the grand symphony of chemistry, pure substances play a pivotal role. They are the characters, the notes, and the musical instruments that combine to create the harmony and complexity of the natural world. As we unravel the secrets of elements and compounds, we deepen our understanding of the universe we inhabit.
Compounds: When Elements Join Forces
In the realm of chemistry, pure substances reign supreme. Among them, compounds stand out as fascinating entities where the boundaries of elements blur, giving birth to new and remarkable substances. A compound is a pure substance composed of two or more elements chemically combined in fixed proportions.
Imagine a grand ballroom filled with countless atoms, each representing a different element. Suddenly, a graceful waltz begins, as these atoms pair up and dance, forming molecules. In the case of compounds, these molecules are composed of two or more different types of atoms, creating a harmonious blend of elements.
The formation of a compound is a testament to the power of chemical bonding. Picture the atoms as tiny magnets, drawn together by opposing charges. They share their electrons, forming invisible bonds that hold the molecule together. These bonds can be diverse, ranging from ionic to covalent, and they determine the unique properties of each compound.
For instance, sodium and chlorine, two highly reactive elements, join forces to create sodium chloride, also known as common salt. The resulting compound is a stable, crystalline solid with entirely different characteristics from its parent elements. Its salty taste and high solubility in water make it an indispensable ingredient in our culinary and industrial worlds.
The world of compounds is vast and diverse, with applications that touch every aspect of our lives. They form the backbone of materials science, providing us with sturdy metals, resilient plastics, and advanced ceramics. In medicine, compounds serve as potent drugs, saving lives and alleviating suffering. And in engineering, they power our engines, lubricate our machines, and protect us from the elements.
So, the next time you sprinkle salt on your food or marvel at the strength of your smartphone, remember the extraordinary dance of elements that gave rise to these indispensable compounds. Their existence is a testament to the transformative power of chemistry and its ability to create materials that shape our world.
Exploring Compounds: When Elements Join Forces
In the enchanting realm of chemistry, where the boundless world of matter unveils its secrets, we embark on an enthralling journey to unravel the mysteries that shroud compounds – fascinating substances that emerge when elements dance together in a harmonious symphony.
Compounds, the very embodiment of chemical unity, are pure substances that originate from the captivating union of two or more distinct elements. They are not mere concoctions of their elemental constituents; rather, they undergo a profound metamorphosis, emerging as unique entities with their own distinctive identities and remarkable properties.
The formation of compounds is orchestrated by the invisible bonds of chemical reactions, which weave together elements with an intricate dance of attraction. These reactions, fueled by the relentless pursuit of stability, drive the rearrangement and combination of elemental atoms, giving birth to a vast array of compounds that grace our world.
The properties of compounds are a tantalizing tapestry woven from the threads of their constituent elements. These substances inherit certain traits from their elemental parents, yet they also possess novel characteristics that stem from their unique atomic arrangements. Compounds possess their own distinct melting points, boiling points, chemical reactivity, and myriad other attributes that set them apart from their elemental components.
The allure of compounds lies not only in their intrinsic elegance but also in their boundless applications that permeate every corner of our lives. From the miracle drugs that heal our bodies to the innovative materials that shape our modern world, compounds play a pivotal role in shaping our existence. They are the driving force behind countless technological advancements, empowering us to harness the wonders of science for the betterment of humanity.
Understanding Compounds: When Elements Join Forces
In the world of pure substances, we encounter a fascinating realm where elements, the fundamental building blocks of nature, come together to form intricate compounds. Compounds are unique substances that embody the power of collaboration, where two or more elements harmoniously combine to create something entirely new.
Imagine a symphony orchestra, where each instrument represents an element. On their own, these instruments may produce beautiful melodies, but when they blend their sounds, they create a rich and captivating masterpiece. Similarly, elements possess distinct properties, but when they unite, they give rise to compounds with remarkable characteristics that surpass the capabilities of their individual components.
The formation of compounds is a testament to the power of chemical reactions. These reactions occur when atoms of different elements rearrange their electrons, forming new and stable bonds. It’s like a dance, where atoms gracefully intertwine to create a new entity with its own unique identity.
Compounds are the architects of our material world. They shape the molecules that comprise our bodies, the drugs that heal us, and the materials that build our homes. Their properties, such as strength, flexibility, and reactivity, stem from the harmonious interplay between their constituent elements.
So, as we navigate the world of pure substances, let us not only marvel at the simplicity of elements but also embrace the complexity and wonder of compounds. They are the result of nature’s exquisite chemistry, a testament to the power of collaboration in the atomic realm.
Related concepts: chemical reactions, chemical bonding, and properties of compounds.
Understanding the Chemistry of Compounds: A World of Combined Elements
In the realm of chemistry, pure substances reign supreme, and among them, compounds stand out as captivating creations. Unlike their simpler counterparts, elements, which are made up of identical atoms, compounds embody the alluring magic of collaboration. When elements join forces, they forge new substances with extraordinary properties that often surpass those of their individual components.
At the heart of compound formation lies chemical reactions, intricate dances of atoms rearranging themselves to form a new molecular tapestry. Chemical bonding, the invisible thread that binds atoms together, weaves this tapestry, determining the shape, stability, and reactivity of the resulting compound. Each compound is a unique blend of elements, its properties sculpted by the alchemy of their union.
From the humble salt that seasons our food to the potent drugs that heal our bodies, compounds play an indispensable role in our daily lives. They form the very fabric of our world, from the materials we build with to the medicines we rely on. Understanding the chemistry of compounds empowers us to harness their power, unlocking a vast potential for innovation and progress.
Subheading: Separating the Atoms from the Mix
IV. Distinguishing Elements and Compounds: Separating the Atoms from the Mix
In the vast realm of chemistry, elements and compounds reign supreme as distinct entities. While both are classified as pure substances, their inner composition tells a captivating tale of difference. Elements, the fundamental building blocks of our universe, consist of only one type of atom. In contrast, compounds are more intricate creations, forged by the union of two or more elements.
Imagine a vibrant mosaic, where each tile represents an element. In this analogy, a compound would be like a masterpiece created by carefully arranging the tiles to form intricate patterns. The atomic composition of elements and compounds is a key differentiator. While elements boast a uniform atomic structure, compounds exhibit a diverse range of atomic arrangements, giving rise to their unique properties.
What truly sets compounds apart is their remarkable ability to form through chemical reactions. These reactions involve a rearrangement and combination of atoms, creating new substances that possess entirely new characteristics. For instance, consider the union of hydrogen and oxygen, two elements that blend together to form water, a compound with life-sustaining properties.
In this dance of atoms, the dance of atoms, chemical bonding plays a pivotal role. Like the invisible threads that bind a construction, chemical bonding holds the atoms of a compound together. The strength and nature of these bonds determine the various properties of compounds, from their stability to their reactivity.
In the grand scheme of things, elements and compounds are indispensable to our world. Elements provide the raw materials for countless industries, from construction to electronics. Compounds, in turn, encompass an astounding array of substances, from the water we drink to the medicines that heal us. Their versatility and importance are a testament to the remarkable power of pure substances and the intricate interplay of atoms that make up our universe.
Distinguishing Elements and Compounds: Separating the Atoms from the Mix
In the realm of pure substances, elements and compounds stand out as captivating entities that shape the world around us. While both belong to this exclusive club, their identities are far from identical. To unravel their differences, we embark on a journey to understand their atomic composition and the transformative power that emerges when elements unite.
Atomic Architecture: Unraveling the Composition
Elements, the very building blocks of nature, are defined by their singular atomic composition. Each element contains atoms of only one type, lending them a distinctive homogeneity that sets them apart. Picture a pristine garden where each flower boasts a uniform color and shape, representing the elemental purity of these fundamental substances.
In contrast, compounds are born from the union of two or more elements, creating a new entity with a unique atomic fingerprint. These substances are like collaborative masterpieces, where elements intertwine and harmonize to give birth to novel creations. A compound’s composition is a testament to the remarkable diversity that arises from the rearrangement and combination of its elemental constituents.
Unique Properties: Unveiling the Essence of Compounds
The fusion of elements in compounds unlocks a realm of extraordinary properties that far surpass the capabilities of their individual components. Compounds possess characteristics that are entirely distinct from their elemental counterparts, as the rearrangement and combination of atoms introduce a plethora of possibilities.
Imagine a painter blending vibrant pigments to create a captivating masterpiece. Similarly, when elements converge to form compounds, their attributes undergo a remarkable transformation. The resulting substance takes on a new identity, with properties tailored to its specific composition. For instance, the combination of hydrogen and oxygen gives rise to water, a life-sustaining elixir that is vastly different from its gaseous constituents.
Elements and compounds, though distinct in their atomic composition, share a common bond as the cornerstone of our material world. Their unique properties and versatility have fueled advancements in science, medicine, and engineering, shaping the very fabric of our civilization.
From the towering skyscrapers that grace our cities to the life-saving medicines that combat disease, pure substances play an indispensable role in our daily lives. Their ability to form new substances, each with tailored properties, has unlocked countless possibilities and continues to inspire scientific breakthroughs that shape our future.
Distinguishing Elements from Compounds: Unraveling the Atomic Composition
In the realm of chemistry, the distinction between elements and compounds holds paramount importance. Understanding their contrasting atomic makeup empowers us to delve deeper into the fascinating world of pure substances.
Elements: The Building Blocks of Nature
Elements stand as the fundamental building blocks of matter, each composed of atoms with identical atomic numbers. These atomic numbers signify the number of protons residing within the atom’s nucleus, which dictate the element’s identity. For instance, all atoms containing six protons are carbon atoms, the foundation of countless organic molecules.
Compounds: When Elements Unite
In contrast, compounds emerge when two or more elements join forces, forming new substances with distinct properties from their constituent elements. This union involves chemical reactions, where electrons are shared or transferred between atoms to create chemical bonds. These bonds hold the atoms together, giving rise to compounds with unique atomic compositions.
Atomic Composition: A Defining Contrast
The key distinction between elements and compounds lies in their atomic composition. Elements exist as pure substances, consisting of only one type of atom. Compounds, however, are composed of two or more different types of atoms chemically bonded together. This fundamental difference profoundly influences their properties.
Unveiling the Properties of Pure Substances
The distinct atomic compositions of elements and compounds manifest in their properties. Elements generally exhibit characteristic physical and chemical properties, such as melting points, boiling points, and reactivity, which are directly related to their atomic structure. Compounds, on the other hand, display properties that differ from their constituent elements due to the rearrangement and combination of atoms during chemical bonding.
Applications: The Power of Pure Substances
Understanding the distinction between elements and compounds is crucial in various scientific disciplines and industries. From materials science to medicine and engineering, the ability to harness the properties of pure substances has led to transformative advancements. For example, in materials science, engineers leverage the unique properties of elements and compounds to create alloys with enhanced strength and durability. In medicine, pharmaceutical scientists utilize the precise composition of compounds to design targeted therapies for specific diseases.
In essence, the world of pure substances unveils a captivating saga of atomic composition and its profound impact on the properties and applications of these substances. By unraveling the distinctions between elements and compounds, we gain invaluable insights into the fundamental building blocks of our universe.
The Unique Properties of Compounds: A Symphony of Elements
In the realm of chemistry, pure substances like elements and compounds play pivotal roles. Elements, the fundamental building blocks of nature, consist solely of atoms of the same type. Compounds, on the other hand, are born from the harmonious union of two or more elements. It’s in this fusion that the magic of compounds unfolds.
Compounds exhibit properties distinct from their constituent elements, a testament to the transformative power of their unique atomic arrangement. This remarkable alchemy stems from the intricate dance of chemical bonding. When elements combine, their atoms share electrons, creating a symphony of attractions that dictate the compound’s characteristics.
For instance, sodium and chlorine, two highly reactive elements, form sodium chloride (NaCl), also known as common salt. This compound, so different from its parent elements, is a stable, crystalline solid essential for life. Its unique properties allow it to dissolve in water, making it an important electrolyte in biological systems.
Another fascinating example is water (H2O). Composed of hydrogen and oxygen, water’s versatile properties make it the elixir of life on Earth. Its ability to dissolve a wide range of substances, its high heat capacity, and its unique surface tension are all attributed to the intricate interactions between its hydrogen and oxygen atoms.
The world of compounds is a vast and wondrous one, with countless examples of their transformative power. From the fuels that power our lives to the pharmaceuticals that heal us, compounds are the architects of our modern world. Their unique properties, born from the rearrangement and combination of elements, continue to inspire awe and innovation, shaping the very fabric of our existence.
The Power of Pure Substances
In the vast tapestry of the natural world, pure substances stand out like radiant beacons, the fundamental building blocks that shape our existence. Elements, the intrinsic essence of nature, are composed of a solitary type of atom, the indivisible units of matter. Hydrogen, the lightest and most bountiful element, whispers through the cosmos, forming the celestial tapestry of stars. Oxygen, the lifeblood of our planet, sustains all living creatures, a testament to the elemental symphony of nature.
Compounds, on the other hand, are born when elements intertwine, their dance of chemical bonding giving rise to new substances with unparalleled properties. Water, a ubiquitous compound, is the elixir of life, quenching the thirst of every living organism. Carbon dioxide, the invisible exhales of living creatures, nourishes the vibrant greenery that adorns our landscapes. Compounds are the architects of our material world, from the steel that forges skyscrapers to the medicines that safeguard our health.
The distinction between elements and compounds is not merely a matter of atomic composition but a testament to the transformative power of chemistry. When elements join forces, they rearrange themselves, forming intricate molecular structures with unique properties that differ vastly from their elemental constituents. Water, for instance, is a polar compound, its molecules possessing a slight electrical charge, which allows it to dissolve a multitude of substances. This remarkable characteristic has made water an indispensable solvent, essential for countless biological and industrial processes.
The power of pure substances is evident in every corner of our world. In materials science, elements and compounds are combined to create advanced materials with extraordinary properties. Carbon nanotubes, composed of pure carbon atoms arranged in a hexagonal lattice, possess remarkable strength and electrical conductivity, making them ideal for applications in electronics and lightweight materials. In medicine, compounds like aspirin and penicillin have revolutionized healthcare, alleviating pain and combating infections, respectively. And in engineering, alloys, such as steel and bronze, are crafted by combining elements to enhance their strength, durability, and versatility.
In essence, pure substances are the unsung heroes of our universe, the architects of our material world and the healers of our bodies. Their intricate dance of bonding and rearrangement gives rise to the myriad of substances that shape our lives, from the air we breathe to the medicines that protect our health. As we delve deeper into the realm of chemistry, may we continue to unlock the transformative power of these elemental building blocks, forging a future where the power of pure substances continues to illuminate our path.
The Power of Pure Substances: Elements and Compounds in Our World
Imagine a world where everything we see and touch is made up of just one type of atom. That’s the realm of elements, the basic building blocks of nature. Compounds enter the picture when different elements team up, forming a new substance with its own unique characteristics.
Elements: The Building Blocks of Nature
Elements are the simplest form of matter, consisting of only one type of atom. Gold, silver, and oxygen are examples of elements that exist in their pure form. The key concept here is that an element cannot be broken down into simpler substances through chemical reactions.
Compounds: When Elements Join Forces
Compounds, on the other hand, are composed of two or more different elements chemically bonded together. Water (H2O) is a classic example, with two hydrogen atoms and one oxygen atom joining forces. Salt (NaCl) is another compound, with sodium and chlorine atoms forming a unique substance.
Distinguishing Elements and Compounds
So, how do we tell elements and compounds apart? Elements are pure substances, composed of only one atom, while compounds are pure substances, formed by the combination of two or more elements. This difference in atomic composition gives each type of substance its distinctive properties.
The Power of Pure Substances
The diversity of elements and compounds is astounding. Elements like carbon form the backbone of life, while silicon is essential for electronics. Compounds like aspirin relieve pain, and fertilizers enhance crop yields.
In materials science, elements like titanium and tungsten create stronger, lightweight alloys. In medicine, compounds like penicillin and vaccines fight infections and protect our health. In engineering, elements like copper and aluminum enable electrical circuits and structures.
Elements and compounds are the fundamental building blocks of our physical world, shaping everything from the stars to the air we breathe. Their diversity and applications are endless, highlighting the incredible power of pure substances in shaping our lives and the world around us.