Unveiling The Nuclear Symphony: The Vital Roles Of Protons And Neutrons In Atoms
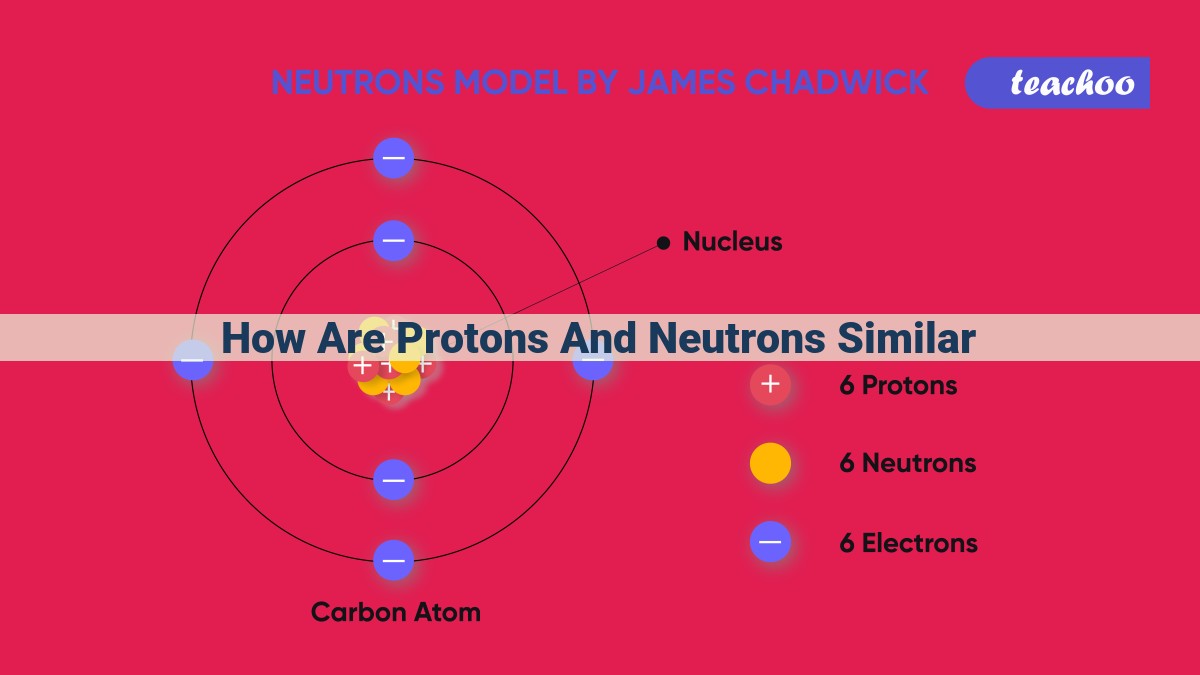
Protons and neutrons share fundamental roles within atoms as nuclear residents. Both particles reside in the nucleus, a dense core containing most of the atom’s mass. Protons carry a positive charge, while neutrons are neutral. This charge difference distinguishes them but does not hinder their collaboration. The strong nuclear force binds protons and neutrons together, overcoming electrostatic repulsion. The unique combination of protons and neutrons defines an element and its isotopes, influencing stability and potential nuclear reactions. Understanding their properties is crucial for grasping atomic structure and nuclear chemistry.
Subatomic Building Blocks
Subatomic Building Blocks: The Foundation of Matter
Embark on an atomic adventure as we delve into the fascinating realm of subatomic particles, the fundamental building blocks of everything around us. From the tiniest microscopic organisms to the vast celestial bodies, all matter is composed of these minuscule wonders.
At the heart of every atom lies the nucleus, a tiny but dense core that harbors protons and neutrons. Protons, with their positively charged nature, contribute to an atom’s identity. They determine the element to which the atom belongs, be it hydrogen, helium, or any of the others on the periodic table.
Neutrons, on the other hand, carry a neutral charge. They act as mediators within the nucleus, balancing the electrostatic repulsion between positively charged protons. Together, protons and neutrons form the bulk of an atom’s mass, creating its atomic mass number.
Nuclear Residents: The Heart of the Atom
In the bustling metropolis of the atom, there exists a compact and enigmatic neighborhood known as the nucleus. This microscopic realm is home to two influential residents: protons and neutrons.
Protons, the nucleus’s positively charged occupants, are the backbone of the atom’s identity. Their number, aptly named the atomic number, determines which element the atom belongs to. Neutrons, on the other hand, are the uncharged companions of protons, contributing to the nucleus’s mass without affecting its electrical nature. Together, these subatomic residents form a densely packed core that accounts for the majority of the atom’s weight.
Within the nucleus, protons and neutrons coexist in a peculiar dance. Despite their opposing charges, a powerful force, known as the strong nuclear force, binds them together. This force is so potent that it overcomes the electrostatic repulsion between protons, keeping the nucleus intact.
The ratio of protons to neutrons in the nucleus is crucial for the atom’s stability. When this ratio is balanced, the nucleus is stable. However, an imbalance can lead to unstable nuclei that undergo radioactive decay, altering the number of protons or neutrons and potentially leading to the formation of different elements.
Understanding the properties and interactions of protons and neutrons is essential for comprehending the very foundations of atomic structure and nuclear chemistry. These subatomic residents play a pivotal role in shaping the identities of elements, governing their stability, and influencing the intricate world of nuclear reactions.
Charge and Mass Contribution: The Building Blocks of Atomic Nuclei
Deep within the heart of every atom lies a tiny, dense core known as the nucleus. This enigmatic realm is home to two fundamental particles: protons and neutrons. Protons, adorned with a positive electric charge, act as the atomic nucleus’s charged residents. Neutrons, on the other hand, possess a neutral charge, making them silent companions within the nuclear abyss.
Together, protons and neutrons contribute the bulk of an atom’s mass. Each proton weighs approximately the same as each neutron, and the combined mass of protons and neutrons forms the nucleus’s mass. This dense concentration of mass gives the nucleus its incredible stability, anchoring it at the atom’s center.
Despite their mass similarities, protons and neutrons play distinct roles. Protons contribute a positive charge to the nucleus, while neutrons maintain a neutral charge. The balance between proton and neutron numbers determines an element’s identity and the types of isotopes it can form.
The Uniting Force: The Strong Nuclear Force
Imagine a microscopic universe where the tiniest particles dance around in a complex ballet. Protons and neutrons, the building blocks of the atomic nucleus, are in a constant struggle against their inherent repulsion. They’re like tiny magnets that should be pushing each other apart.
But there’s a hero in this microscopic world: the strong nuclear force. This invisible force is so powerful that it overrides the electrostatic repulsion between protons, bringing them and the neutral neutrons together.
The strong nuclear force is like the superglue of the atomic nucleus, holding these positively charged particles in place. Without it, all the protons would fly apart, and the nucleus, the heart of the atom, would collapse.
The strength of the strong nuclear force is mind-boggling. It’s about a trillion times stronger than the electromagnetic force that governs our everyday interactions. This incredible strength is what keeps the nucleus incredibly dense, packing a tremendous amount of mass into a tiny space.
The dance of protons and neutrons within the nucleus is a delicate balance. Too few or too many neutrons can weaken the strong nuclear force, making the nucleus unstable. When this happens, the nucleus can decay or rearrange itself, releasing energy in the form of radiation.
Understanding the strong nuclear force is crucial for unraveling the mysteries of the atomic nucleus and nuclear chemistry. It’s a force that unites the tiniest particles, shaping the very foundations of matter as we know it.
Nuclear Fingerprint: The Defining Identity of Elements
Imagine the nucleus of an atom as a bustling metropolis, where each resident plays a crucial role in shaping the very essence of the atom. Among these nuclear inhabitants are two key players: protons and neutrons. Protons, with their positive charge, are the powerhouses of the nucleus, while neutrons, despite their neutral charge, provide stability and balance.
The ratio of protons to neutrons in an atom’s nucleus is like a unique fingerprint, creating a distinct identity for each element. This fingerprint determines not only the element’s chemical properties but also its existence in various forms known as isotopes.
Isotopes are atoms of the same element but with different numbers of neutrons. Think of it this way: they’re like siblings in a family, sharing the same number of protons (the family name) but having different numbers of neutrons (the individual characteristics).
For instance, carbon, an essential building block of life, exists as three isotopes: carbon-12, carbon-13, and carbon-14. All three have six protons, but carbon-12 has six neutrons, carbon-13 has seven, and carbon-14 has eight. These different neutron numbers give each isotope unique properties and applications.
Understanding the nuclear fingerprint of an element is not just an academic pursuit. It has profound implications in fields like medicine, energy, and forensics. By manipulating the isotopes of certain elements, scientists can develop drugs that target specific diseases, create more efficient nuclear reactors, or determine the age of ancient artifacts.
Ultimately, the nuclear fingerprint serves as a testament to the intricate workings of the atomic world, revealing the symphony of protons and neutrons that shape the building blocks of our universe.
Stability and Radioactivity
In the realm of atomic structure, the delicate balance between protons and neutrons plays a pivotal role in determining the stability of atomic nuclei. While protons carry a positive charge, neutrons remain neutral. This charge imbalance creates an inherent electrostatic force between protons, which would otherwise repel each other. Yet, within the confines of the nucleus, a powerful force holds these protons together, preventing their dispersion: the strong nuclear force.
This strong nuclear force, a testament to the intricate tapestry of the universe, overcomes the repulsive forces between protons. As a result, it ensures the integrity of atomic nuclei, allowing for the construction of elements and the existence of matter as we know it.
However, this stability is not absolute. In certain nuclei, an imbalance can arise, leading to a deviation from the harmonious equilibrium of protons and neutrons. When this occurs, the nucleus teeters on the brink of instability, triggering a series of nuclear reactions in an attempt to restore its delicate balance.
These nuclear reactions, often involving the emission of particles such as alpha or beta particles, are collectively known as radioactivity. Radioactive nuclei emit these particles to achieve a more stable state, a state where the balance between protons and neutrons is restored.
In the world of nuclear chemistry, understanding the stability and radioactivity of atomic nuclei is paramount. It opens doors to unraveling the mysteries of radioactive isotopes, their applications in medicine and energy production, and the fundamental forces that govern the building blocks of our universe.
The Nucleus: Core Concepts in Atomic Structure
At the heart of every atom lies a miniature universe, its nucleus, a realm where matter’s most fundamental building blocks reside. Protons, positively charged particles, and neutrons, their neutral counterparts, dance together within this nucleus, their collective weight shaping the atom’s mass.
The nucleus is the central command center, where the atom’s identity is forged. The number of protons determines the element itself, while the balance of protons and neutrons gives each isotope its unique fingerprint.
Despite their proximity, these charged protons should repel each other. Yet, amidst this chaotic symphony, a powerful force intervenes, the strong nuclear force. Like a cosmic glue, it binds these protons and neutrons together, overcoming the repulsive electrostatic forces that threaten to tear the nucleus apart.
This dance of protons and neutrons influences the atom’s stability. When this delicate balance is disturbed, the nucleus may undergo nuclear reactions, altering the number of protons and neutrons, and potentially leading to the emission of radiation.
Understanding the properties of protons and neutrons is paramount for unraveling the mysteries of atomic structure and nuclear chemistry. These core concepts lay the foundation for exploring the intricacies of matter, from the smallest of particles to the vastness of the cosmos.