Proton Number: Unraveling An Element’s Identity And Chemical Properties
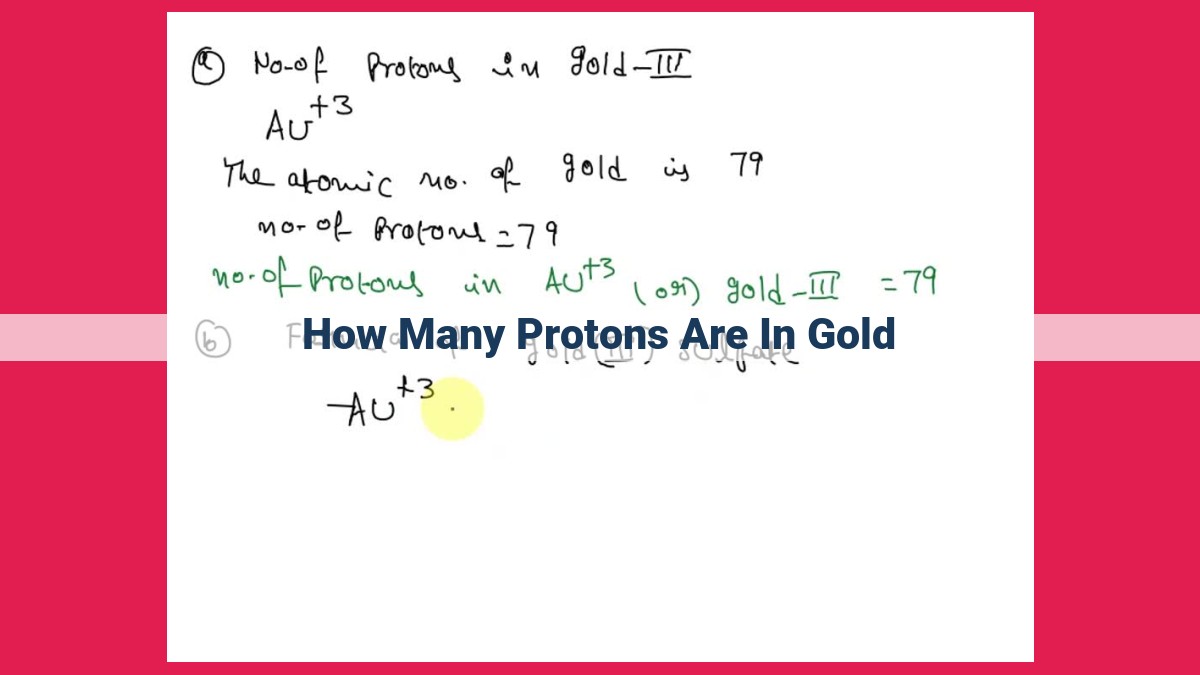
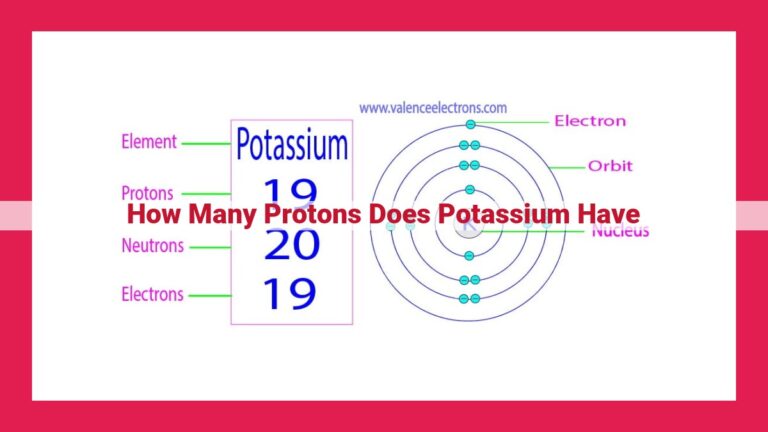
The proton number, also known as the atomic number, determines an element’s identity. It represents the number of protons in an atom’s nucleus, which in turn influences the element’s chemical properties. The atomic number of gold (Au) is 79, which indicates that a gold atom has 79 protons in its nucleus. This proton number categorizes gold as an element in the 11th group and 6th period of the periodic table, contributing to its characteristic chemical behavior and unique properties among other elements.
Decoding the Essence of Elements: Proton Number and Atomic Number
In the realm of chemistry, understanding the fundamental characteristics of elements is paramount. Two crucial concepts that define an element’s identity are the proton number and atomic number. Let’s delve into their significance and unveil how they shape the very foundation of our atomic world.
Proton Number: The Heart of an Element’s Identity
At the core of an atom lies its nucleus, a tiny powerhouse that houses positively charged protons and neutral neutrons. The proton number of an element is simply the number of protons residing within its nucleus. This number is unique to each element, distinguishing it from all others.
Atomic Number: The Ultimate Identifier
The atomic number of an element, often denoted by the symbol Z, is synonymous with its proton number. It represents the number of protons in an atom’s nucleus and is the primary determinant of an element’s chemical identity. Elements with different atomic numbers are distinct chemical entities with varying properties.
The Unbreakable Bond: Proton Number and Atomic Number
The proton number and atomic number are intrinsically linked, forming an inseparable pair. They provide the blueprint for an element’s properties and behavior. By knowing the proton number, we can precisely identify the element and predict its position on the periodic table, a tabular arrangement of elements based on their atomic numbers.
Unveiling the Periodic Table’s Secrets
The periodic table serves as a roadmap to the elemental kingdom, organizing elements in a logical manner based on their atomic numbers. Each element occupies a specific location on the table, dictated by its proton number. This arrangement reveals patterns and relationships between elements, aiding in the prediction of their properties and reactivities.
From Atomic Structure to Nuclear Charge
The proton number not only defines an element’s identity but also plays a crucial role in shaping its atomic structure. Protons, along with electrons, determine an atom’s overall charge and chemical behavior. The number of protons determines the positive nuclear charge, which influences the atom’s ability to attract or repel other particles. This charge is fundamental to understanding chemical bonding and interactions between atoms.
In conclusion, the proton number and atomic number serve as the cornerstones of our understanding of elements. They define an element’s identity, shape its atomic structure, and determine its place on the periodic table. By unraveling the mysteries of these fundamental concepts, we gain deeper insights into the fascinating world of chemistry and the tapestry of matter that surrounds us.
Element Identity and the Periodic Table
The periodic table is a brilliant tool that scientists use to organize and understand the chemical elements. It’s a chart that reveals the fascinating relationships and patterns among these elements, making it easier for us to predict their properties and behavior.
One of the most critical factors that determines an element’s identity is its atomic number. This number is like a unique fingerprint for each element. It represents the number of protons found in the nucleus of an atom. Protons have a positive charge, and the atomic number, therefore, determines the positive charge of the nucleus.
The periodic table is organized based on the atomic numbers of the elements. Elements with the same atomic number have the same number of protons and belong to the same vertical column, known as a group. They share similar chemical properties due to their identical electron configurations.
For instance, gold, with an atomic number of 79, is located in Group 11 of the periodic table. This means that all the elements in Group 11 have 79 protons and share similar chemical characteristics, such as being highly malleable and ductile.
By understanding the concept of atomic number and its relationship with the periodic table, scientists can quickly identify elements, predict their properties, and explore their potential applications. This knowledge has been instrumental in the development of new technologies and materials, contributing to our technological advancements.
Nuclear Charge and Proton Number: Unveiling the Heart of the Atom
Diving into the Depths of an Atom
Imagine an atom as a miniature universe, with its own unique structure and components. At the very core lies the nucleus, a tiny, dense region that houses the most fundamental particles of matter: protons and neutrons. Protons carry a positive electric charge, while neutrons are electrically neutral.
The Power of Protons
The proton number, also known as the atomic number, is the number of protons found within an atom’s nucleus. This vital number holds immense significance as it determines an element’s identity. Elements are the building blocks of matter, each with unique properties and characteristics. The periodic table, a tabular arrangement of elements, is organized based on their atomic numbers.
The Proton’s Influence on Atomic Properties
The proton number not only identifies an element but also influences atomic properties. The positive charge of protons attracts electrons, which orbit around the nucleus in specific energy levels. The number of protons, or the nuclear charge, determines the number of electrons an atom can accommodate, thereby shaping its chemical reactivity and behavior.
In summary, the proton number is a fundamental property of atoms that defines their identity, nuclear charge, and atomic properties. Understanding this concept unlocks the door to deciphering the complexities of the subatomic world and unraveling the mysteries of matter.