Propane: Characteristics And Properties For Efficient Fuel Usage
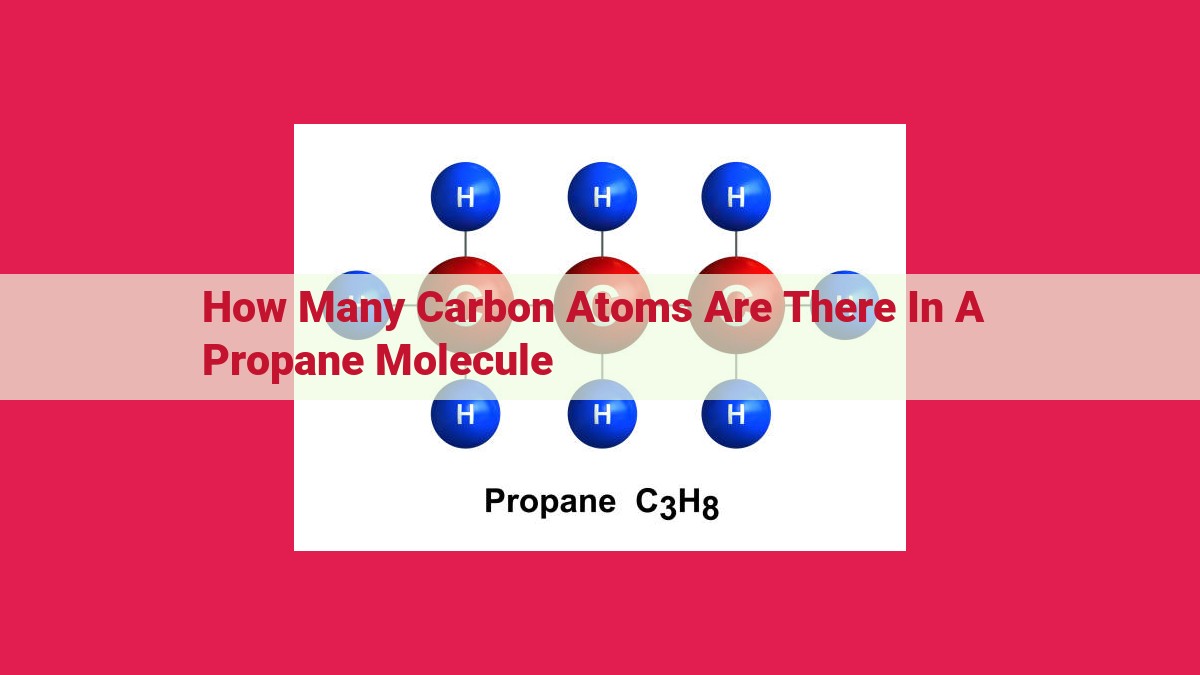
- Propane, a hydrocarbon commonly used as fuel, contains three carbon atoms.
- Propane is an alkane, meaning it consists of carbon and hydrogen atoms bonded by covalent bonds.
- The molecular formula of propane (C3H8) indicates that it has three carbon atoms.
- The number of carbon atoms determines the length of the propane molecule and its molecular weight.
Unveiling the Hidden Atoms: Exploring the Carbon Count in Propane
In the vast realm of chemistry, molecules hold countless secrets, awaiting our discovery. Today, we embark on a captivating journey to uncover one such secret: the number of carbon atoms in propane. Prepare to be amazed as we delve into the fascinating world of molecular structures and unravel the mysteries that lie within.
Propane, a ubiquitous hydrocarbon molecule, plays a pivotal role in our everyday lives. It fuels our homes, powers our vehicles, and even propels our outdoor adventures. But beneath its seemingly simple exterior lies a hidden truth, a numerical enigma waiting to be solved. How many carbon atoms does propane contain? Join us as we embark on a thrilling quest to unveil this molecular mystery.
Understanding Propane’s Chemical Structure
Delving into the Realm of Alkanes
The world of chemistry is full of fascinating molecules, each with its unique properties and characteristics. Among these molecules, we encounter alkanes, a group of organic compounds that form the building blocks of many fuels and other essential substances. Propane, the focus of our exploration today, is a prime example of an alkane.
The Molecular Architecture of Propane
To unravel the mysteries of propane’s structure, we need to delve into the concept of covalent bonds. Covalent bonds are chemical bonds formed when two atoms share electrons, creating a strong attraction that holds them together. In propane, these covalent bonds play a crucial role in shaping its molecular architecture.
Carbon, the central element in propane, forms the backbone of the molecule. It readily bonds with other carbon atoms, forming chains or rings. In the case of propane, three carbon atoms join together to create a straight chain, resembling a miniature railroad track.
Hydrogen, a versatile element, serves as propane’s companion. Each carbon atom eagerly welcomes three hydrogen atoms, forming a total of eight hydrogen atoms attached to the carbon chain. These hydrogen atoms act like tiny satellites, orbiting their carbon nuclei.
A Diagrammatic Representation
To fully grasp the three-dimensional nature of propane, let’s visualize it using a molecular diagram. Imagine a carbon chain resembling a line segment with three branches stemming from it. Each branch represents a hydrogen atom, and the three carbon atoms are connected by invisible covalent bonds. This simple diagram provides a glimpse into the intricate structure of propane, revealing the harmonious arrangement of its atoms.
Significance of the Number of Carbon Atoms
In the realm of chemistry, the number of carbon atoms in a molecule plays a crucial role in shaping its properties and behavior. In the case of propane, a common fuel, the presence of three carbon atoms holds profound significance.
The chain of carbon atoms acts as the backbone of the propane molecule. The length of this chain is directly influenced by the number of carbon atoms. A higher number of carbon atoms results in a longer chain, leading to increased molecular size.
Additionally, the molecular weight of propane is closely tied to the number of carbon atoms. Molecular weight is the sum of the atomic weights of all the atoms in a molecule. As each carbon atom has an atomic weight of 12, the presence of three carbon atoms in propane contributes significantly to its molecular weight.