Potassium: An Alkali Metal With A Unique Atomic Structure And 19 Protons
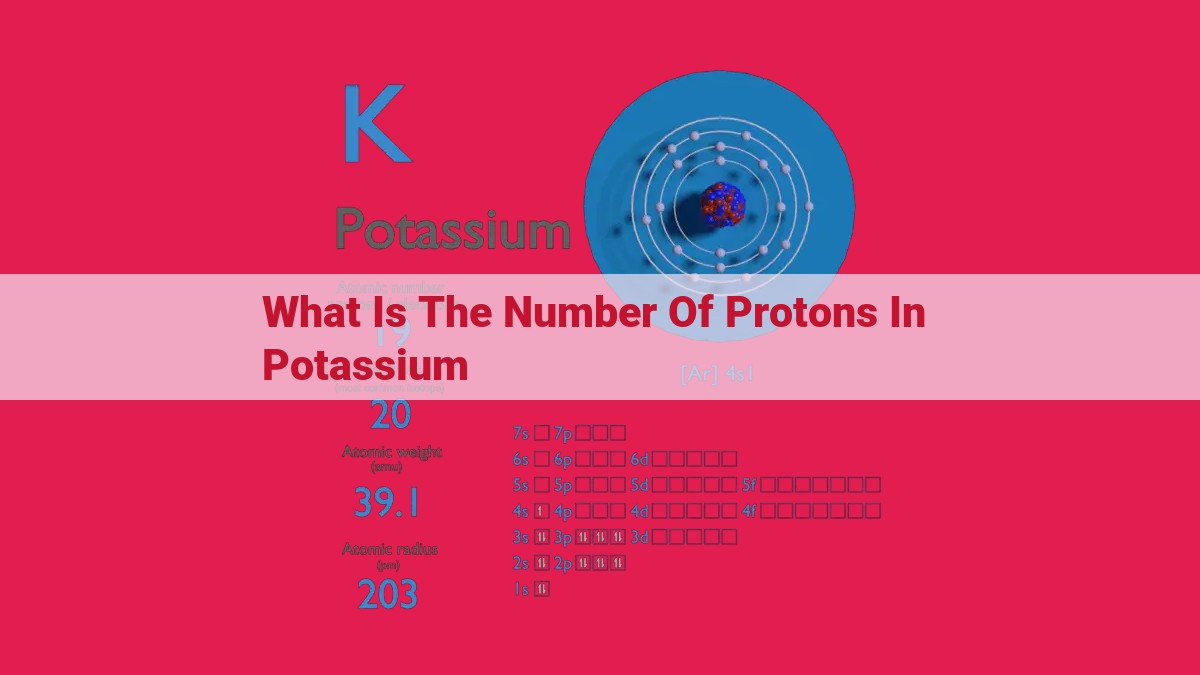
Potassium, an alkali metal in Group 1 of the periodic table, possesses a unique atomic structure. Its atomic number defines its identity, revealing the number of protons (positively charged particles) in its nucleus. The number of protons in an atom of potassium is equal to its atomic number, which is 19. This signifies the presence of 19 protons in the potassium nucleus, contributing to its overall positive charge and balancing the negative charges carried by the 19 electrons orbiting the nucleus, ensuring electrical neutrality.
Atomic Number of Potassium: Unveiling the Number of Protons
Dive into the fascinating realm of atomic structure and uncover the secrets of potassium’s atomic number. The periodic table, a treasure trove of elemental information, holds the key to understanding the unique identity of potassium.
Potassium resides in Group 1 of the periodic table, a group characterized by elements with a single electron in their outermost shell. This electron, known as a valence electron, plays a pivotal role in potassium’s chemical interactions.
Delve into the atomic structure of potassium, like exploring a microscopic world. At the heart of every potassium atom lies its nucleus, a dense core containing both protons and neutrons. Protons, with their positive charge, are crucial for determining an element’s atomic number.
The atomic number is a unique identifier, a fingerprint for each element. It represents the number of protons within the nucleus. For potassium, this atomic number is 19. This means that every potassium atom, regardless of where it’s found, contains exactly 19 protons.
The number of protons in an atom has profound implications. It dictates the element’s electrical neutrality, ensuring that the overall charge of the atom remains balanced. In potassium’s case, the 19 protons are counterbalanced by 19 electrons, resulting in a neutral charge. This balance is essential for potassium’s stability and its ability to interact with other elements.
Number of Protons vs. Electrons: Exploring Electrical Neutrality
In the vast expanse of the atomic realm, protons and electrons play a delicate dance, maintaining a harmony of electrical neutrality. Every element in the periodic table, including the enigmatic potassium, adheres to this fundamental principle.
Electron configuration, a concept entwined with the atomic number, describes the arrangement of electrons within an atom. Potassium, with its atomic number of 19, boasts 19 electrons circling its nucleus. These electrons occupy specific energy levels, each with its own unique number of orbitals.
Among these 19 electrons, a single valence electron resides in potassium’s outermost energy level. This lone ranger plays a pivotal role in chemical interactions, forming bonds with other atoms to achieve a more stable electronic configuration.
Just as the number of protons in an atom defines its identity, so too does the balance between protons and electrons determine its overall charge. In the case of potassium, the 19 protons in its nucleus are matched by 19 electrons. This delicate equilibrium ensures that potassium maintains an overall charge of zero, a state known as electrical neutrality.
Electrical neutrality is not merely a matter of balancing numbers; it’s a fundamental requirement for the stability of matter. Atoms with an imbalance between protons and electrons become charged ions, creating a realm of chemical reactivity. Understanding the number of protons in potassium and its relationship with electrons is therefore crucial for comprehending its atomic structure and chemical behavior.
Determining the Number of Protons in Potassium
Unveiling the Atomic Heart of Potassium
To determine the number of protons in potassium, we embark on a scientific journey that unveils the very essence of this remarkable element. At the heart of every potassium atom lies its atomic nucleus, a compact realm teeming with subatomic particles: protons and neutrons.
Protons: The Pillars of Identity
The atomic number of an element defines its unique position on the periodic table. This number corresponds precisely to the number of protons residing within the nucleus. Each proton carries a positive electrical charge, balancing the negative charges of the surrounding electrons.
Potassium’s Position in the Periodic Table
Potassium, a member of Group 1, is the 19th element on the periodic table. This placement signifies that every potassium atom possesses 19 protons. This number is fundamental to understanding potassium’s atomic structure and chemical properties.
Calculating the Number of Protons
The atomic number provides a straightforward method for determining the number of protons in potassium. Since potassium’s atomic number is 19, it follows that each potassium atom contains 19 protons. This unwavering number is immutable, defining potassium’s unique atomic identity.
Electrical Neutrality: Balancing the Scales
The presence of 19 protons in potassium creates an overall positive charge in the nucleus. To maintain electrical neutrality, potassium atoms acquire 19 electrons. These electrons orbit the nucleus, their negative charges precisely counterbalancing the positive charges of the protons. This delicate balance ensures the potassium atom’s stability.
Understanding the number of protons in potassium is crucial for comprehending its atomic structure and chemical behavior. The 19 protons within each potassium atom determine its unique position on the periodic table, establish its electrical neutrality, and influence its reactivity with other elements. This knowledge empowers us to unravel the mysteries of potassium’s atomic world and appreciate its significance in the intricate tapestry of chemistry.