Unlocking The Secrets Of Potassium: Symbol, Properties, And Significance
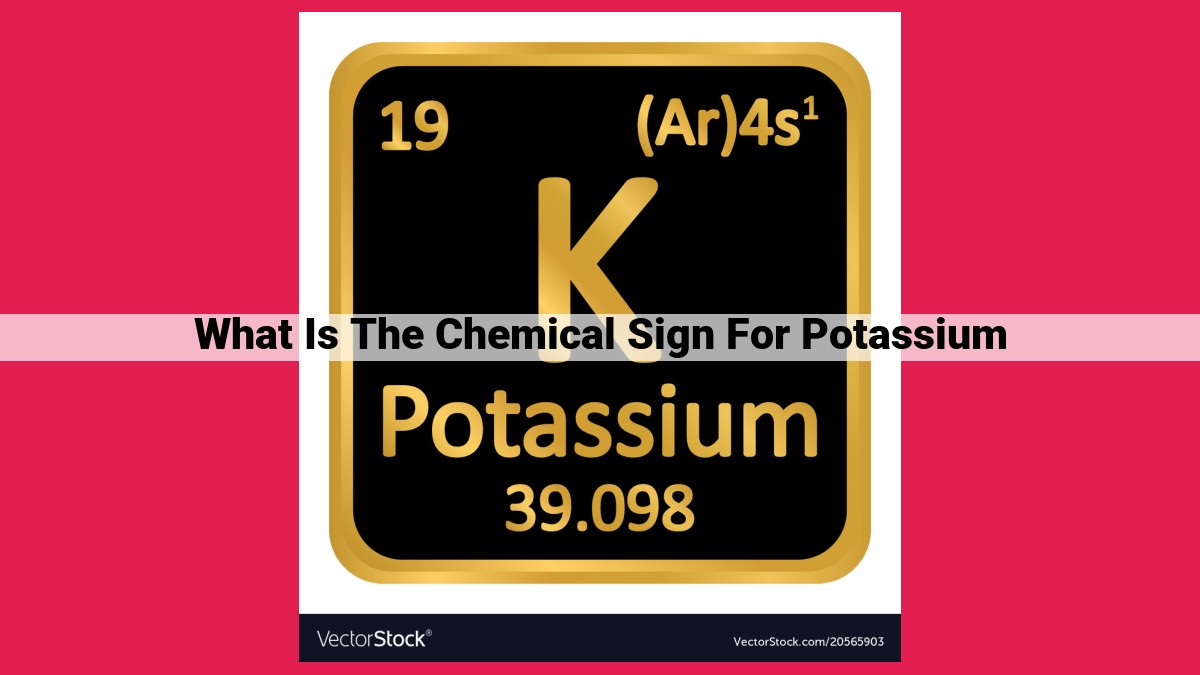
Potassium, an essential mineral for life, is denoted by the chemical symbol “K”. Originating from the Latin word “kalium”, “K” has become the universally recognized abbreviation for potassium in the periodic table. Potassium has an atomic number of 19, indicating 19 protons and 19 electrons in its neutral state. Its chemical formula is simply “K”, signifying its elemental nature. Potassium can form ions, either K+ by losing an electron or K- by gaining one. The symbol “K” is extensively employed in scientific writing to represent potassium in equations, formulas, and diagrams.
Introduction:
- Brief overview of potassium and its importance as an essential mineral.
Potassium: The Essential Mineral Represented by the Universal Symbol “K”
In the realm of essential minerals, potassium stands tall, orchestrating a symphony of vital functions within our bodies. From regulating fluid balance to transmitting nerve signals, this mineral plays an indispensable role in our overall well-being.
Unveiling the Origins of “K”
The enigmatic chemical symbol “K” that represents potassium holds a fascinating history. It traces its roots back to the Latin word kalium, a nod to the Arabic term al-qali, which referred to a plant yielding potash, a rich source of this mineral.
Universally Recognized, Globally Utilized
The symbol “K” has become a universally accepted shorthand for potassium in the periodic table of elements. Scientists, educators, and researchers worldwide employ this symbol to convey the presence of this essential mineral in their work. Its simplicity and ease of recognition make it an invaluable tool in the field of chemistry.
Peering into the Atomic Realm
The atomic number of potassium, proudly displayed as “19,” reveals the presence of 19 protons and 19 electrons in its neutral state. This atomic makeup places potassium within the realm of alkali metals, elements known for their high reactivity and a tendency to form positively charged ions.
Striving for Balance: The Chemical Formula
In its purest form, potassium exists as the element itself, represented by the simple chemical formula “K.” This formula encapsulates the element’s unaltered state, free from any chemical bonds or associations with other elements.
The Versatility of Potassium Ions
Potassium atoms possess a remarkable ability to transform into ions, shedding or acquiring electrons to achieve stability. The resulting positively charged ions, known as “K+,” play a crucial role in maintaining fluid balance and transmitting nerve impulses, while negatively charged “K-” ions find applications in various industrial processes.
The Chemical Symbol of Potassium: A Tale of Origins
The enigmatic symbol K that represents potassium holds a fascinating story, rooted deep in the annals of science and etymology. Allow us to delve into the origins of this symbol, a journey that transports us to the very heart of chemical nomenclature.
Potassium’s chemical symbol, K, is a testament to its Latin heritage. In the 18th century, a Swedish chemist named Torbern Bergman isolated a new alkali that he named kali, derived from the Arabic word al-qali, meaning “plant ashes.” This alkali exhibited remarkable properties, particularly its ability to neutralize acids.
As scientists further investigated kali, it became evident that it was not a single element but rather a compound, a salt containing potassium. With the advent of modern chemistry and the development of the periodic table, potassium was finally recognized as a unique element, and the symbol K was assigned to it. This symbol served as a permanent marker of potassium’s identity, forever linking it to its Latin roots.
The Ubiquitous Symbol “K”: A Tale of Universal Recognition
In the realm of scientific nomenclature, the chemical element potassium reigns supreme with its enigmatic symbol, “K.” This unassuming letter has become the universally accepted symbol for potassium in the periodic table of elements, its story echoing the element’s fundamental importance in the natural world.
The origin of “K” can be traced back to the Latin word “kalium,” a moniker bestowed upon potassium by the Swedish chemist Jöns Jakob Berzelius in 1813. Berzelius’ choice of “kalium” paid homage to the Arabic word “al-qalyah,” which referred to the plant ashes that were a rich source of potassium.
As the scientific community delved deeper into understanding the elements, the need for a standardized system of symbols arose. In the 1800s, scientists proposed various symbols for potassium, including “Po” for “potassium” and “K” for “kalium.” However, it was not until the first International Congress of Chemistry in 1860 that “K” emerged as the universally accepted symbol for potassium, a decision that stands to this day.
The symbol “K” has become deeply ingrained in the scientific vocabulary, effortlessly conveying the presence and behavior of potassium in chemical equations and scientific literature. In research papers, on laboratory benches, and in classrooms, “K” serves as a succinct and universally recognized shorthand, bridging the gap between scientists and facilitating the seamless exchange of scientific knowledge.
The universal recognition of “K” as potassium’s symbol is a testament to the power of international collaboration and the shared desire to create a coherent and accessible language for the scientific community. This iconic symbol not only represents potassium but also embodies the spirit of scientific unity and the enduring quest for knowledge.
The Atomic Makeup of Potassium: Exploring the Inner Workings of the Essential Mineral
As we delve into the realm of chemistry, we encounter a myriad of elements, each holding a unique place in shaping our world. Among them stands potassium, an indispensable mineral vital for numerous physiological processes. Beyond its biological significance, understanding the atomic structure of potassium unveils a captivating narrative that intertwines history, physics, and chemistry.
Atomic Number 19: A Tale of 19 Protons
Nestled in the periodic table’s Group 1, potassium reigns as an alkali metal. Its atomic number, 19, signifies the presence of 19 protons within the nucleus, the central core of the atom. These positively charged protons wield the defining force that characterizes potassium and distinguishes it from other elements.
A Balance of Charge: Electrons in Orbit
Complementing the protons, an equal number of negatively charged electrons dance around the nucleus. In a neutral potassium atom, we find 19 electrons diligently orbiting the nucleus in specific energy levels. This harmonious balance between protons and electrons renders potassium electrically neutral, maintaining its stable state.
The Potassium Atom: A Blueprint for Life
The atomic structure of potassium serves as a blueprint for its remarkable properties. Its single valence electron – the electron occupying the outermost energy level – grants potassium high reactivity, allowing it to readily form ions. This ionic nature of potassium plays a crucial role in maintaining electrical gradients and electrolyte balance within living organisms, making it essential for proper nerve function, muscle contraction, and hydration.
In conclusion, the potassium atom is an intricate and fascinating entity. Its atomic number of 19, along with its specific electron arrangement, endows potassium with unique characteristics that make it an indispensable element for life on Earth. Understanding the atomic makeup of potassium provides a deeper appreciation for its biological importance and the intricate workings of the natural world.
Chemical Formula:
- Explain that the chemical formula for potassium is also “K,” indicating its elemental nature.
Understanding the Chemical Formula of Potassium: K
In the vast realm of chemistry, elements are the building blocks that form the world around us. One such crucial element is potassium, an indispensable mineral that plays a vital role in our bodies and various scientific processes. To unravel the mysteries of this element, we delve into its unique chemical formula: K.
Potassium’s Elemental Nature
Potassium proudly stands as an element in its own right, earning its place on the periodic table with the chemical symbol K. This elemental nature means that potassium exists in its pure form, uncombined with any other elements. It has the atomic number 19, signifying that its nucleus holds 19 protons and orbits contain 19 electrons.
The Chemical Formula “K”
The chemical formula of an element is a concise representation of its composition. For potassium, this formula is simply K. This straightforward notation highlights the element’s uncombined state. Potassium does not typically bond with other elements to form compounds. Instead, it prefers to remain in its pure, elemental form.
Potassium Ions: A Special Case
While potassium typically exists as an element, it can sometimes form ions. Ions are atoms that have gained or lost electrons, resulting in an electrical charge. Potassium ions have the chemical formula K+ or K-. The + indicates a positively charged ion, while the – denotes a negatively charged ion.
Uses of the Chemical Formula K
The chemical formula K is widely used in scientific writing, equations, and diagrams to represent potassium. It serves as a shorthand notation for the element, simplifying the communication of complex chemical concepts.
The chemical formula K encapsulates the essence of potassium as an element. It reveals potassium’s pure, uncombined nature and its occasional ability to form ions. Understanding this formula unlocks a deeper appreciation for the enigmatic world of chemistry and the fascinating role of potassium in our lives and the universe.
Potassium Ions: The Electrical Force of the Atom
Potassium’s journey in the world of chemistry is an extraordinary one. It starts with its ability to transform into something entirely different—ions. Ions are atoms that have gained or lost electrons, giving them an electrical charge.
Potassium atoms have a particular talent for shedding electrons, becoming positively charged potassium ions (K+). These ions play a crucial role in our bodies, helping to regulate fluid balance and nerve impulses.
On the other hand, potassium can also gain electrons, becoming negatively charged potassium ions (K-). These ions are not as common as their positively charged counterparts, but they still have their place in the world of chemistry.
Ions are the unsung heroes of chemistry. They allow atoms to interact with each other in ways that would be impossible if they remained neutral. Potassium ions are just one example of the many ions that play a vital role in our world.
The Universal Symbol of Potassium: A Story of Its Significance
Potassium, an essential mineral for life, plays a crucial role in various bodily functions. Its chemical symbol, K, carries a fascinating story of universal recognition and scientific significance.
Origin of “K”: A Latin Connection
The symbol “K” for potassium originates from the Latin word “kalium,” which refers to the substance known as “potash,” a potassium-rich compound. The word “kali” itself is derived from Arabic, where it denoted “alkali.” This connection highlights the historical and linguistic journey of potassium’s symbol.
Universal Recognition: A Periodic Symbol
In the periodic table of elements, “K” is the universally accepted symbol for potassium. This common language of science allows researchers, educators, and enthusiasts alike to seamlessly understand and communicate about potassium across borders and disciplines.
The Potassium Atom: A Tale of Electrons
Potassium possesses an atomic number of 19, indicating the presence of 19 protons in its nucleus. In its neutral state, it has 19 electrons, equally distributed in energy levels. This atomic structure defines the unique properties of potassium.
Chemical Formula: Elemental Simplicity
The chemical formula for potassium is simply “K.” This elemental symbol represents its pure form, unbonded with other elements. The “K” symbol serves as a shorthand notation in chemical equations and scientific writing.
Potassium Ions: Losing and Gaining
Potassium atoms can undergo ionization, a fascinating process where they lose or gain electrons. By losing an electron, they become positively charged potassium ions (K+). Conversely, by gaining an electron, they transform into negatively charged potassium ions (K-). These ions play significant roles in biological processes, such as nerve impulse transmission.
Uses of the Symbol “K”: A Scientific Language
The “K” symbol permeates scientific literature, equations, and diagrams, representing potassium’s presence and involvement. Whether in describing chemical reactions, discussing ionic interactions, or highlighting physiological functions, the symbol “K” serves as a concise and universal way to convey information about potassium.