Potassium: Chemistry, Isotopes, And Nuclear Composition For Seo
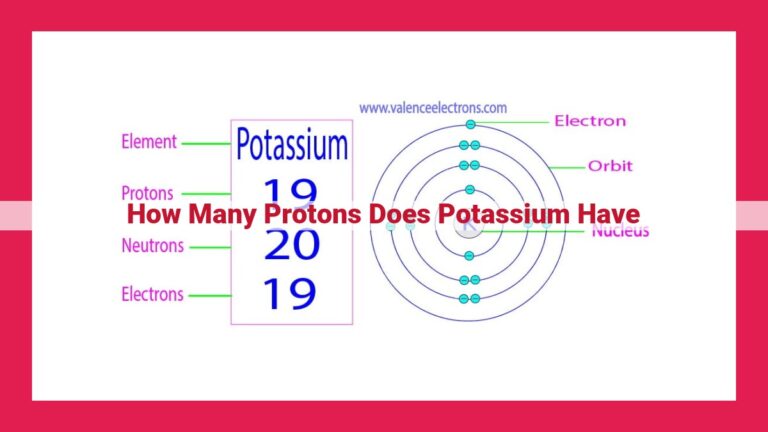
Potassium, a highly reactive alkali metal (Group 1), has an atomic number of 19 and a mass number of 39. The difference between its mass number and atomic number reveals 20 neutrons in the nucleus. Isotopes of potassium have varying neutron counts while maintaining its unique element identity.
Potassium: An Alkali Metal
- Explain that potassium is a highly reactive metal belonging to Group 1 of the periodic table.
- Discuss its atomic weight (39.0983 u) and chemical symbol (K).
Understanding Potassium: The Alkali Metal
Embarking on a scientific adventure, let’s unravel the mysteries surrounding potassium, an element that plays a crucial role in maintaining the balance of our bodies.
Belonging to Group 1 of the periodic table, potassium is a highly reactive metal with an atomic weight of 39.0983 atomic mass units and the chemical symbol K. Its atomic number, representing the number of protons in its nucleus, is 19.
Unveiling the Atomic Structure of Potassium
Journey into the heart of an atom, where subatomic particles reside. Neutrons, found alongside protons in the atomic nucleus, play a vital role in ensuring atomic stability. As these particles combine, they form the atom’s mass number, which represents the total number of protons and neutrons. In the case of potassium, its mass number is 39, indicating the presence of a total of 39 subatomic particles.
Unraveling Isotopes: Variations Within Elements
Atoms of the same element can exist in different forms known as isotopes. These isotopes have identical atomic numbers but varying mass numbers. The difference in mass numbers is attributed to a variation in their neutron count.
Determining Potassium’s Neutron Count
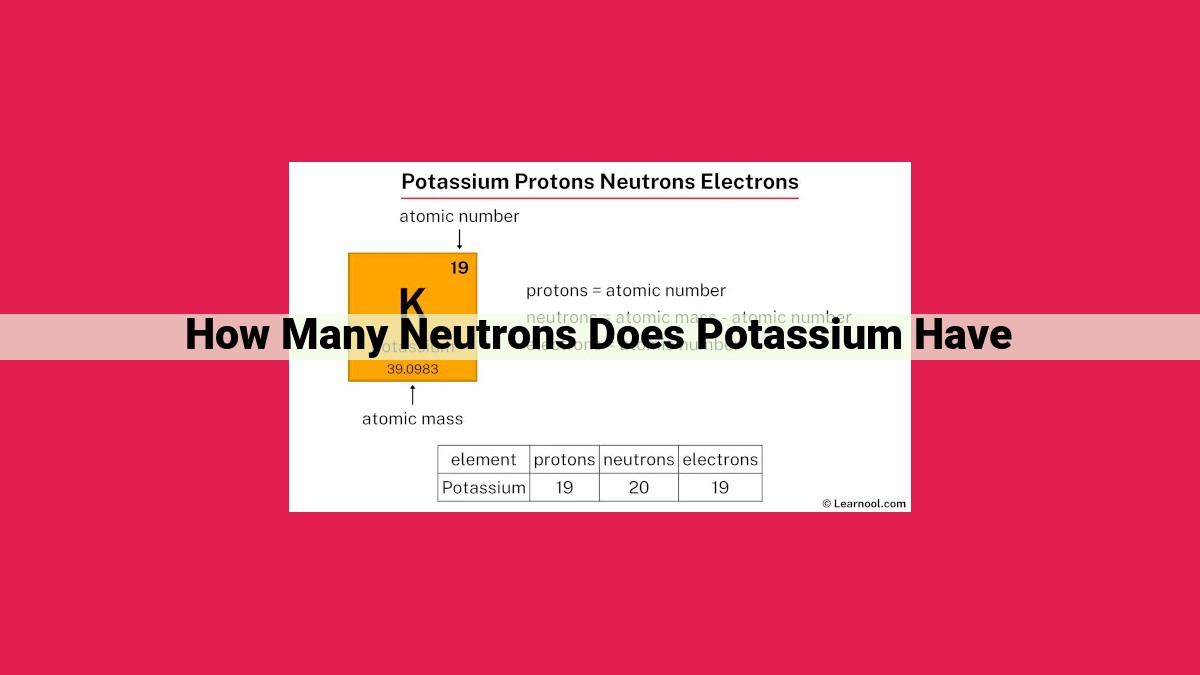
Understanding the fundamental relationship between atomic number, mass number, and neutron count is key to unraveling potassium’s neutron count. By subtracting potassium’s atomic number (19) from its mass number (39), we arrive at 20, revealing the number of neutrons present in its nucleus.
Neutrons: The Unsung Heroes of Atomic Stability
In the bustling metropolis of an atom’s nucleus, a cast of subatomic characters plays a pivotal role in shaping its destiny. Among them, neutrons stand as unsung heroes, silently contributing to the stability and harmony of this microscopic realm.
Meet the Neutron: The Nucleus’ Silent Guardian
Neutrons are humble particles, lacking the electrical charge of protons and the graceful spin of electrons. They reside deep within the nucleus, the atom’s central command center, alongside their positively charged counterparts.
The Strong Nuclear Force: The Unshakeable Bond
Despite their neutral nature, neutrons play a crucial role in maintaining the integrity of the nucleus. The strong nuclear force, a powerful glue-like interaction, binds protons and neutrons together, overcoming the repulsive forces between protons. Without this force, atomic nuclei would crumble apart like fragile glass.
Atomic Stability: A Balancing Act
The number of neutrons in an atom directly influences its stability. Too few neutrons, and the nucleus becomes unstable, prone to radioactive decay. Too many neutrons, and the atom becomes sluggish and unwieldy. The optimal balance of protons and neutrons ensures the atom’s longevity and stability.
Atomic Number: Unlocking the Identity of Elements
We live in a vast world composed of countless substances and materials, each with unique properties and characteristics. Understanding the fundamental building blocks of these substances is crucial to unraveling their nature and behavior. One of the most important factors in determining an element’s identity is its atomic number.
In the heart of every atom resides the nucleus, a tiny, dense core that houses the atom’s protons and neutrons. Protons are positively charged particles, while neutrons are electrically neutral. The atomic number of an element is simply the number of protons found in its nucleus.
For instance, let’s take the element potassium, a highly reactive metal that plays essential roles in our bodies. Potassium has an atomic number of 19, which means each potassium atom contains 19 protons. This unique number of protons distinguishes potassium from all other elements, making it a distinct entity with specific properties.
The atomic number is a fundamental characteristic of an element that determines its position on the periodic table. Elements are arranged in the periodic table based on their atomic numbers, creating a logical order that reveals patterns and trends in their properties.
Understanding atomic numbers is not only crucial for unraveling the mysteries of elements but also for comprehending the vast tapestry of chemical reactions and interactions that shape our world. Every chemical reaction involves the rearrangement of atoms, and the atomic numbers of the elements involved dictate the outcome and properties of the resulting compounds.
So, next time you hear about an element, remember the importance of its atomic number. It’s the atomic fingerprint that sets each element apart and unlocks the secrets to its identity and behavior.
Mass Number: Protons and Neutrons Combined
- Explain that the mass number is the sum of protons and neutrons in an atom’s nucleus.
- Provide the mass number of potassium as 39, indicating a total of 39 particles.
Mass Number: A Tale of Protons and Neutrons
Just like every living creature is made up of different components, each atom has its own unique “recipe” consisting of subatomic particles. Among them are protons and neutrons, the building blocks of an atom’s nucleus. When these two particles come together, they form the mass number, a crucial piece of information that defines an element.
For our protagonist, potassium, the mass number is 39. This number reveals that the potassium nucleus is a bustling metropolis of 39 particles—protons and neutrons working together to create the element’s identity.
Imagine potassium’s nucleus as a cozy little house. Within this house live protons, each carrying a positive charge, and neutrons, their neutral counterparts. To determine the number of protons, we need to look at potassium’s atomic number, which is 19. This means that our potassium house has 19 lively protons dancing around its nucleus.
So, how do we find the number of neutrons? It’s a simple subtraction game. We subtract the atomic number (19) from the mass number (39), which gives us 20—the number of neutrons residing in the potassium nucleus.
Now, you might be wondering, “Why is the mass number so important?” Well, it’s like a secret code that tells us about an element’s stability and unique characteristics. By understanding the mass number, scientists can unlock the secrets of isotopes—variations of the same element with different numbers of neutrons—and their impact on the world around us.
Isotopes: The Variants within Elements
In the realm of chemistry, atoms, the building blocks of our universe, possess unique characteristics that define their identity. One crucial aspect is the neutron count, which significantly influences an atom’s behavior. To unravel this intriguing concept, let’s delve into the fascinating world of isotopes.
Isotopes: Unveiling the Hidden Differences
The term “isotope” refers to atoms of the same element that share an identical atomic number (the number of protons). However, these atomic siblings differ in their neutron count. While the atomic number determines an element’s identity, the number of neutrons present in an atom’s nucleus gives rise to variations within the same element.
Maintaining Elemental Identity amidst Neutron Count Fluctuations
Isotopes of an element share the same number of protons and electrons, preserving their chemical properties. As a result, they exhibit identical reactivity and occupy the same position on the periodic table. Despite their varying neutron counts, they remain faithful representatives of their respective elements.
Unraveling Potassium’s Neutron Count
To illustrate the concept further, let’s explore potassium, an element essential for our bodily functions. Potassium’s atomic number is 19, indicating the presence of 19 protons in its nucleus. The element’s mass number (the sum of protons and neutrons) is 39, revealing a total of 39 particles within its core. Subtracting the atomic number from the mass number, we can deduce that potassium has 20 neutrons.
The Impact of Neutron Count on Atomic Stability
The neutron count plays a crucial role in determining the stability of an atom. A balanced neutron-to-proton ratio contributes to a stable atomic nucleus. This delicate equilibrium ensures that atomic nuclei remain intact, preventing them from disintegrating or undergoing radioactive decay.
In summary, isotopes are siblings within the elemental family, sharing the same atomic number but differing in neutron count. They maintain the chemical characteristics of their parent element while introducing subtle variations in atomic mass. Understanding isotopes is paramount for unraveling the complexities of atomic structure and its implications in various fields of science.
Potassium’s Neutron Count: Unveiling the Hidden Truth
In the vast expanse of the universe, where matter intertwines in an intricate dance, elements play a pivotal role. Elements, composed of atoms, are the building blocks of everything around us. And within these atoms lies a fascinating world of subatomic particles, each with its unique properties and contributions.
Among these elements, potassium stands out as a key player in maintaining the balance of life. This highly reactive metal, belonging to Group 1 of the periodic table, holds a wealth of secrets waiting to be unveiled.
One of these secrets lies in the enigmatic realm of neutrons – subatomic particles nestled within the nucleus of an atom. Neutrons, along with protons, provide the atom with its mass and stability. They dance around the nucleus, held captive by the strong nuclear force, ensuring the atom’s structural integrity.
To unravel the neutron count of potassium, we must first understand the concept of atomic number and mass number. Atomic number refers to the number of protons in an atom’s nucleus – a defining characteristic that determines the element’s identity. Mass number, on the other hand, represents the combined count of protons and neutrons in the nucleus.
In the case of potassium, its atomic number is 19, indicating the presence of 19 protons in its nucleus. Its mass number is 39, signifying a total of 39 particles – both protons and neutrons – within the nucleus.
So, how do we determine the number of neutrons in potassium? It’s a simple mathematical equation:
- Neutron count = Mass number – Atomic number
Plugging in the values for potassium, we get:
- Neutron count = 39 – 19
- Neutron count = 20
Therefore, potassium possesses 20 neutrons, contributing to its overall stability and mass. This delicate balance of particles within the nucleus allows potassium to fulfill its vital roles in biological processes and industrial applications.
By unraveling the neutron count of potassium, we delve deeper into the enigmatic world of atoms and their intricate inner workings. This knowledge empowers us to comprehend the complex interactions within the natural world and appreciate the profound impact of these subatomic particles on our existence.