Potassium: Unveiling The Secrets Of Atomic Identity Through Atomic Number
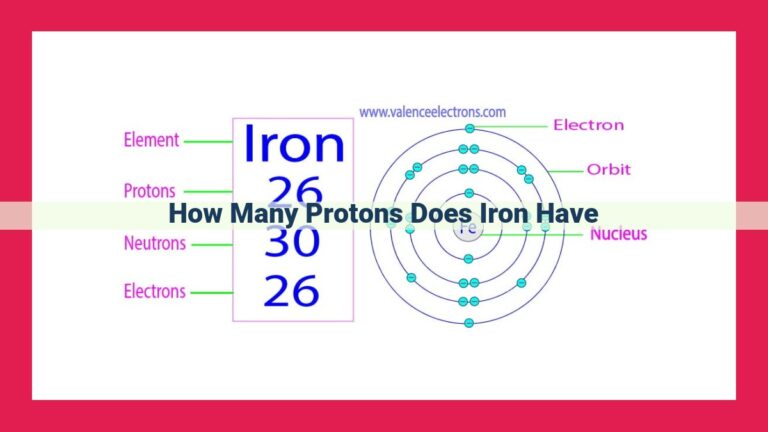
Potassium, an alkali metal, possesses a unique atomic identity defined by its atomic number, which signifies the number of protons in its nucleus. Every potassium atom consistently harbors 19 protons, the same number as its electrons, resulting in a neutral electrical charge. The atomic number not only determines the number of protons but also serves as a cornerstone for understanding an atom’s overall mass and the intriguing realm of isotopes, where variations in neutron count offer insights into the diverse forms of an element.
Unveiling the Secrets of Potassium’s Atomic Structure: A Journey to the Heart of an Atom
Have you ever wondered about the fundamental building blocks of the world around us? The answer lies in the realm of atoms, and in this blog post, we’ll embark on a captivating journey to explore a specific element: potassium. We’ll unravel its atomic secrets, focusing on a fundamental question: How many protons does potassium have?
To understand this, we need to delve into the concept of atomic number, a unique identifier for each element. The atomic number represents the number of protons in the atom’s nucleus, the heart of the atom. This number is crucial as it determines an element’s chemical properties and behavior.
Now, let’s turn our attention to potassium, an alkali metal known for its high reactivity. It’s an essential mineral for our bodies, playing a vital role in regulating nerve impulses and muscle function. Intriguingly, the atomic number of potassium is 19, revealing that each potassium atom possesses 19 protons in its nucleus.
Protons, along with neutrons and electrons, form the subatomic particles that constitute atoms. Protons, carrying a positive electrical charge, reside within the nucleus, while electrons, with their negative charge, orbit the nucleus. In a neutral potassium atom, the number of protons is balanced by an equal number of electrons, resulting in an overall neutral electrical charge.
Beyond protons, understanding isotopes adds another layer of complexity to the atomic world. Isotopes are variations of the same element with different neutron counts. While the number of protons defines an element, the number of neutrons can vary, resulting in isotopes of the same element with varying masses. For example, potassium has three naturally occurring isotopes, each with 19 protons but varying numbers of neutrons.
As we conclude our atomic expedition, we return to our initial question: How many protons does potassium have? The answer is resounding: 19 protons grace the nucleus of every potassium atom. This fundamental understanding of potassium’s atomic structure deepens our appreciation for the intricacies of the building blocks that make up our world.
Atomic Number and Protons
In the vast world of chemistry, the atomic number of an element holds immense significance. It acts as a unique fingerprint, identifying each element and distinguishing it from all others. The atomic number is essentially the number of protons found within the nucleus of an atom.
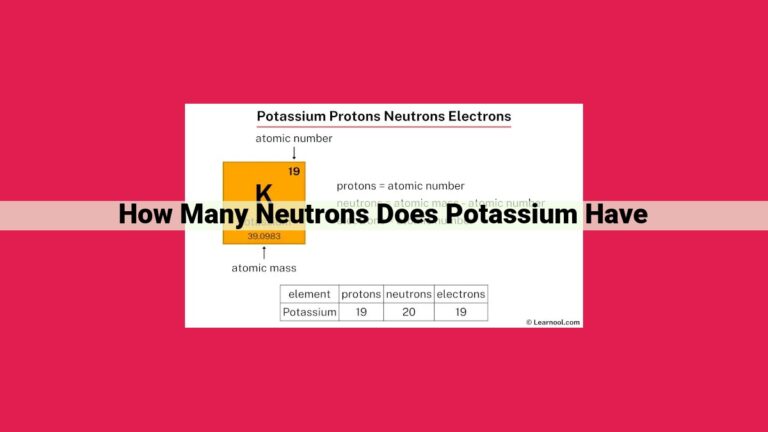
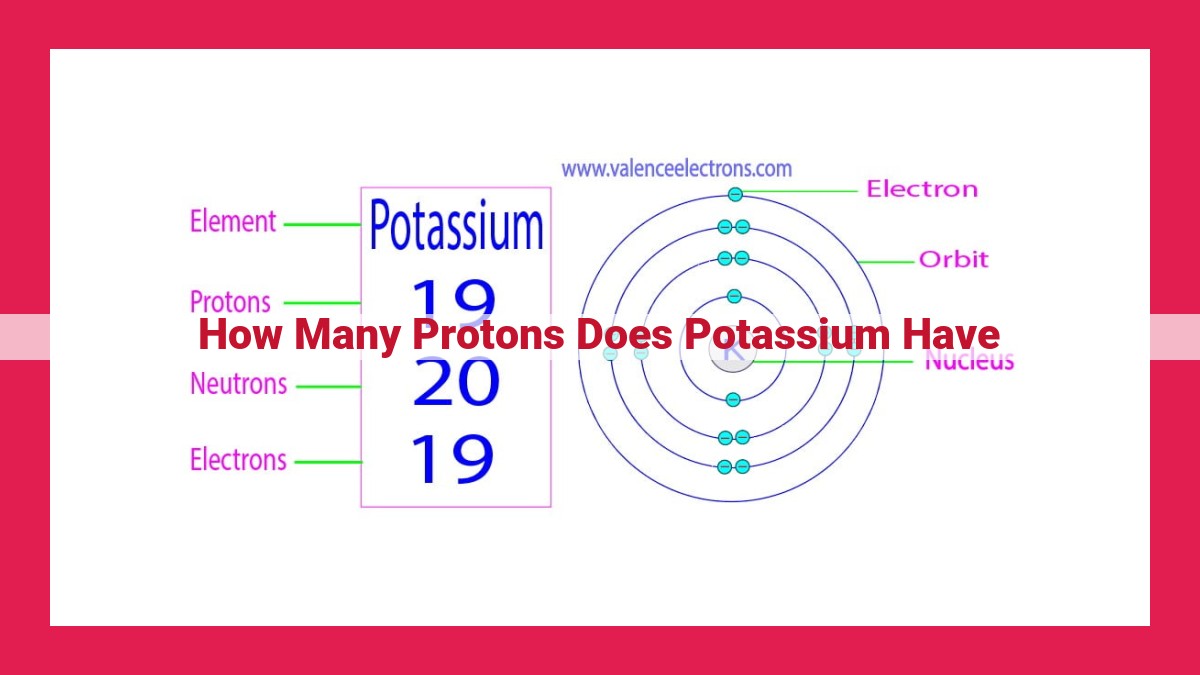
Think of it this way: each element has a specific number of protons. For example, potassium (K), an essential electrolyte in our bodies, has an atomic number of 19. This means that every potassium atom has 19 protons in its nucleus.
The atomic number is not just a random number; it plays a crucial role in determining the chemical properties of an element. Protons are positively charged subatomic particles, and their number influences the element’s attraction to electrons. Electrons, which are negatively charged, orbit the nucleus. The interplay between protons and electrons establishes the chemical bonds that form the basis of all matter.
Therefore, the atomic number serves as a fundamental characteristic of every element, dictating its identity and guiding its interactions in the world of chemistry.
Potassium: An Alkali Metal with 19 Protons
In the vast expanse of the periodic table, potassium resides as an essential alkali metal, renowned for its unique properties. With an atomic number of 19, potassium atoms possess 19 protons. But what does this mean and why is it significant? Let’s delve into the atomic structure of potassium to unravel the mystery.
Potassium, a soft, silvery-white metal, belongs to Group 1 of the periodic table, known as the alkali metals. These elements are highly reactive, readily losing an electron to achieve a stable configuration. Potassium’s atomic number is its defining characteristic, indicating the number of protons within its nucleus.
The number of protons in an atom is directly related to its identity. Protons are subatomic particles with a positive electric charge, residing in the atom’s core alongside neutrons. Each element is uniquely identified by its atomic number, which corresponds to the number of protons in its neutral state.
In the case of potassium, its atomic number of 19 indicates that each potassium atom contains 19 protons in its nucleus. This number of protons determines the element’s chemical properties, as the number of electrons surrounding the nucleus must balance the positive charge of the protons.
In a neutral potassium atom, the number of electrons equals the number of protons. This balance ensures that the atom has no net electric charge. The electrons orbit the nucleus in specific energy levels, determining the atom’s chemical reactivity and bonding behavior.
Understanding the number of protons in an atom is crucial for comprehending the periodic table and the behavior of elements. Potassium’s 19 protons define its identity and shape its properties, making it an essential component in various biological and industrial applications.
How Many Protons Does Potassium Have? A Comprehensive Guide
Protons in Potassium
Potassium, an alkali metal, is the 11th element on the periodic table, with an atomic number of 19. Atomic number is a crucial concept, as it signifies the number of protons within an atom’s nucleus. Every potassium atom, regardless of its isotopic variations, possesses precisely 19 protons.
The nucleus, the heart of an atom, houses the protons and neutrons. Protons carry a positive electric charge, while neutrons carry no charge. In a neutral potassium atom, the number of protons is perfectly balanced by the number of electrons, which reside outside the nucleus. This balance ensures that the atom maintains an overall neutral charge.
The arrangement of protons within the nucleus determines the element’s identity and many of its chemical properties. Potassium’s 19 protons distinguish it from other elements and define its unique atomic structure and behavior. Understanding this fundamental aspect of potassium provides a solid foundation for exploring its chemistry and its vital role in biological processes.
Unveiling the Secrets of Potassium: A Journey into Its Proton Count
Embark with us on a captivating quest to unravel the enigmatic realm of potassium, where we seek to unveil the secrets of its atomic makeup. Join us as we delve into the world of protons, embarking on a journey to unravel the very foundation of this intriguing element.
Atomic Symphony: The Tale of Protons and Atomic Numbers
In the realm of chemistry, each element possesses a unique identity, a fingerprint that distinguishes it from all others. This defining characteristic is known as the atomic number, a numerical value that signifies the number of protons residing within the heart of its atoms. Protons, tiny particles charged with a positive electrical force, serve as the cornerstone of an atom’s nucleus, the central hub of its atomic structure.
Potassium’s Aqueous Abode: An Alkali Metal with a Twist
Among the elements that grace our periodic table, potassium proudly stands as an alkali metal, a group renowned for their exuberant reactivity and kinship with water. These elements eagerly embrace water’s embrace, reacting with it to form a symphony of bubbles, each a testament to their unbridled nature.
Unveiling Potassium’s Proton Count: A Number of Significance
Our journey culminates in the revelation of potassium’s proton count, the very essence of its atomic identity. Drawing upon its atomic number, we uncover that potassium possesses 19 protons, a number that defines its very nature. These protons, nestled snugly within the nucleus, orchestrate the atom’s symphony, shaping its interactions with the world around it.
Protons: The Pillars of Atomic Mass
Protons play a pivotal role in determining the mass of an atom, their collective weight contributing significantly to the atom’s overall heft. Each proton, with its unwavering mass, adds to the atom’s mass, influencing its physical properties.
Exploring Isotopes: Variations in Neutron Numbers
In the realm of atoms, there exist variations known as isotopes. Isotopes are essentially variations of the same element, sharing the same atomic number but differing in their neutron count. Neutrons, neutral particles residing alongside protons within the nucleus, subtly alter the atom’s mass while leaving its proton count unchanged.